
Effect of oral anticoagulation therapy in atrial fibrillation patients with a history of intracranial hemorrhage: a systematic review and meta-analysis
Introduction
Atrial fibrillation (AF) increases the risk of stroke and thromboembolism five-fold (1). The use of oral anticoagulants (OACs), either vitamin K antagonists (VKAs) or direct oral anticoagulants (DOACs), has been demonstrated to reduce stroke, systemic embolism (SE) and mortality in AF patients when compared to control or placebo treatments (2). However, one of the most disabling and life-threatening complication of OACs is spontaneous intracranial hemorrhage (ICH) (3), although the risk is lower with DOACs (4). Results of most randomized clinical trials were not stratified by the history of ICH. This leads to a therapeutic dilemma as to whether starting or permanently avoiding anticoagulation is the best long-term strategy for AF patients after ICH. Clinicians have to weigh the risk of thromboembolism against the risk of recurrent ICH (5,6). Individual studies have attempted to address this challenge but have not yet been able to provide clear guidance on this issue because of conflicting results. Due to the current paucity of high-quality evidence and the absence of definitive guidelines in this area (7), the effectiveness and safety of restarting OACs after ICH remain unclear.
This is a systematic review and meta-analysis of observational studies as well as the latest randomized controlled trials (RCTs) to evaluate the effectiveness and safety of restarting OACs in ICH survivors with AF. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-582/rc) (8).
Methods
Literature search
PubMed, Embase, Cochrane Library, and ClinicalTrials.gov were systematically searched from the inception of each database till December 29, 2021, under MeSH terms and random words. Keywords used to query the databases included: ‘atrial fibrillation’, ‘intracranial hemorrhage or intracerebral hemorrhage’, and ‘oral anticoagulants’. No linguistic restrictions were applied. Searching strategies are listed in the Supplementary file (Appendix 1). Additional records were procured by a hand-search of the references of primitive literature so as to not miss any eligible studies.
Definitions of outcomes
Our outcome measures were ischemic stroke (IS), IS or SE, and all-cause death for the evaluation of effectiveness; and recurrence of ICH, and major bleeding for the assessment of safety.
All of the studies identified outcomes according to the International Classification of Diseases (ICD-9 or ICD-10) diagnosis codes. Major bleeding event was defined according to the International Society on Thrombosis and Hemostasis criteria, including fatal bleeding and/or symptomatic bleeding in a critical area of organ (e.g., intracranial, intraspinal, intraocular, and retroperitoneal) and/or bleeding causing a decrease of ≥20 g/L (≥1.24 mmol/L) in the hemoglobin level or leading to transfusion of ≥2 U of whole blood or red cells (9).
Eligibility criteria and study selection
All published studies comparing oral anticoagulation therapy versus no anticoagulation in AF patients who had survived a non-traumatic ICH were included. Specifically, the criteria for inclusion were as follows: (I) design: prospective or retrospective observational cohorts, RCTs; (II) participants: non-traumatic ICH survivors with persistent or paroxysmal AF as indication for anticoagulation treatment with ICH including intracerebral, subarachnoid, and subdural hemorrhage; (III) intervention: OACs, including VKA or DOACs, regardless of the type, dosage and time interval of OAC prescription for stroke prevention; (IV) control: avoiding OAC after surviving ICH; (V) Outcome measures: long-term outcomes including IS, IS or SE, all-cause death, ICH recurrence and major bleeding events ≥3 months after exposure to OACs or avoiding OACs. For those studies that considered 3 different types of antithrombotic medication exposure (OACs, antiplatelet agents, and no antithrombotic medication), the latter two categories combined were regarded as the no-OAC group in this study. Studies were excluded if at least one of the following situations applied: (I) no relevant data provided (e.g., unfinished studies, reviews, commentaries, case reports, small case series); (II) studies focused on traumatic ICH; (III) single-arm studies without a comparison group; (IV) studies without follow-up or report of outcomes.
Two of the authors (Mei Liu and Yue Hou) carried out the initial selection separately, first based on titles and abstract and then on the full text of all possible candidate studies, according to the criteria above. Wherever there was a disagreement in the lists of studies to be included that the two authors produced separately, the whole research team met for discussions till a consensus was eventually reached.
Data extraction and study quality assessment
Data were extracted independently by two of the authors (Mei Liu and Yue Hou) using a common extraction form. Study characteristics including the following data were documented: first author, year of publishing, study design, demographic and clinical characteristics of the patients, sample size, type of hemorrhage, type of OACs, CHA2DS2-VASc and HAS-BLED scores, timing of OAC start after hemorrhage, and follow-up duration. The quality of the studies was assessed according to the Cochrane handbook (10).
Statistical analysis
All statistical analyses were performed using Review Manager (Version 5.2) and Stata (Version 12.0). The numbers of events and sample size of each group were collected. The odds ratio (OR) with a 95% confidence interval (CI) was calculated for each included study, and then pooled by a random-effect model using the Mantel-Haenszel (M-H) method. Subgroup analysis was performed based on the type of study (non-RCT, RCT). The Cochrane Q tests and I2 statistics were used to evaluate heterogeneity, where I2>50% indicated significant heterogeneity. If heterogeneity was detected, single-variable meta-regression was performed to explore underlying factors. The method of excluding one study at a time was used for sensitivity analysis. Publication bias was assessed using the funnel plots and further calculated by the egger’s tests, with P<0.05 considered an indicator of statistical significance.
Results
Study selection
The flow chart of document retrieval was presented in Figure 1. A total of 2,453 articles from four databases and citation searching were evaluated. After initial screening based on title and abstract, and after research reports and review articles were excluded, the full texts of 39 articles from databases and 2 articles from citation searching were assessed for eligibility. Two of the authors then assessed them separately, and 30 were excluded due to the following reasons: (I) 11 excluded because of a difference in their study objectives with ours; (II) 6 excluded because of an absence of a comparison group; (III) 7 excluded because of lack of data about endpoint; (IV) one feasibility study excluded because of inadequate sample size; and (V) 5 excluded as unfinished studies. Finally, 11 studies were included in our current meta-analysis: 2 completed RCTs (11,12) (APACHE-AF, NCT02565693 and SoSTART, NCT03153150) and 9 observational studies [2 prospective observational (13,14) and 7 retrospective studies (15-21)].
Study characteristics
This meta-analysis covered a total of 18,115 patients (43.6% female) who had AF and a history of ICH from 11 studies. A total of 2,818 (15.6%) of these patients received OACs, and 15,297 (84.4%) did not. Two studies (11,15) included only patients with intraparenchymal hemorrhage as the index ICH, while others widened coverage to include subdural and subarachnoid hemorrhages. Three studies (13,16,18) enrolled patients who received VKAs as the OAC agent, one RCT (11) used apixaban and the other seven studies included patients that used DOACs or VKA as the anticoagulation therapy. For the evaluation of ischemic stroke or SE, all-cause death, 10 studies were eligible (11-15,17-21). There were 10 studies included for the evaluation of ICH recurrence (11-13,15-21). For the evaluation of ischemic stroke (11,12,15,16,18) and major bleeding (11,13-15,18,19,21), 5 and 7 studies were included respectively. Characteristics of the included studies were described in detail in Table 1.
Table 1
| Study | Location | Design | Type of hemorrhage | Total number | Age, mean/median, y | Female, % | HAS-BLED, median | CHA2DS2-VASc, median | FU, medium, mo | Time internal to resumption, medium (SD/IQR) | OAC Group | No-OAC Group | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Therapy | Number | Therapy | Number | ||||||||||||
| Abrantes 2022, (15) | Portugal | Retrospective observational | Spontaneous intracerebral hemorrhage | 95 | 75.2 | 48 | 4 | 4 | 24 | N/A | DOAC/VKA | 40 | No-OAC | 55 | |
| Chao 2016, (16) | Taiwan | Retrospective observational | Spontaneous ICH | 12,917 | 74.7 | 43 | N/A | 6 | 39.6 | N/A | VKA | 1,154 | APA/no-ATM | 11,763 | |
| Nielsen 2015, (17) | Denmark | Retrospective observational | OACs-associated ICH | 1,752 | 78 | 38 | 3.2 | 3.9 | 12 | 34 d | DOAC/VKA | 303 | APA/no-ATM | 1,449 | |
| Nielsen 2017, (13) | Denmark | Prospective observational | Spontaneous ICH | 1,325 | 76.8 | 43.3 | 3.6 | 4.1 | 9.2 | 81.3 (62.8) d | VKA | 377 | No-OAC | 948 | |
| Park 2016, (18) | Korea | Retrospective observational | ICH | 428 | 68.4 | 65.9 | 3.5 | 3.3 | 39.5 | 117.5 (235.7) d | VKA | 254 | No-OAC | 174 | |
| Perreault 2019, (19) | Canada | Retrospective observational | ICH | 683 | 83 | 53.1 | 2.6 | 3.9 | 12 | N/A | DOAC/VKA | 260 | No-OAC | 423 | |
| Poli 2018, (14) | Italy | Prospective observational | ICH | 146 | 77 | 39.7 | N/A | 4.2 | 18 | 1–3 months | DOAC/VKA | 55 | APA/no-ATM | 91 | |
| Sadighi 2020, (20) | US | Retrospective observational | OACs-associated ICH | 93 | 76.2 | 46 | N/A | 4 | 26 | 56 (52.5) d | DOAC/VKA | 38 | No-OAC | 55 | |
| Salman 2021, (12) | UK | RCT, open-label | Spontaneous ICH | 203 | 79 | 36 | 2 | 4 | 14.4 | 115 [49–265] d | DOAC/VKA | 101 | No-OAC | 102 | |
| Schreuder 2021, (11) | Netherland | RCT, open-label | OACs-associated intracerebral hemorrhage | 101 | 78 | 46 | N/A | 4 | 22.8 | 46 [21–71] d | Apixaban | 50 | No-OAC | 51 | |
| Wu 2021, (21) | Taiwan | Retrospective observational | OACs-associated ICH | 372 | 71 | 52.1 | 2.5 | 4.2 | 12 | Within 6 weeks | DOAC/VKA | 186 | No-OAC | 186 | |
HAS-BLED score includes poorly controlled hypertension (1 point), abnormal renal (1 point), abnormal liver function (1 point), prior stroke (1 point), prior major bleeding or predisposition (1 point), elderly (age >65; 1 point), and concomitant drugs with antiplatelet effect (anti-platelets or NSAIDs, 1 point), and excessive alcohol usage (1 point). CHA2DS2-VASc score: a score for predicting the risk of stroke or thromboembolism in atrial fibrillation, based on congestive heart failure (1 point); hypertension (1 point); age ≥75 years (2 points); diabetes (1 point); previous stroke, transient ischemic attack, or thromboembolism (2 points); vascular disease (1 point); age 65–74 years (1 point); and sex category (1 point for female). FU, follow-up; SD, standard deviation; IQR, interquartile range; OAC, oral anticoagulants; N/A, not available; DOACs, direct oral anticoagulants; VKAs, vitamin K antagonists; ICH, intracranial hemorrhage; APA, anti-platelet agent; ATM, antithrombotic medication; RCT, randomized controlled trial; NSAID, nonsteroidal anti-inflammatory drug.
Study results
Effectiveness outcomes of OAC therapy vs. no-OAC therapy
IS
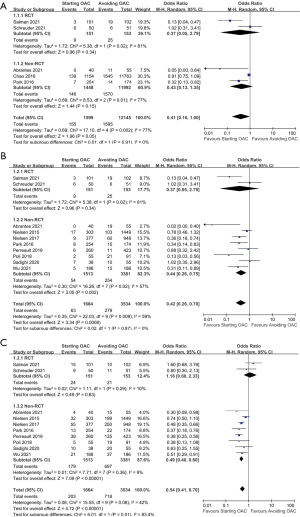
A total of 5 studies compared the occurrence of IS among patients who received OAC therapy and patients who avoided it. Neither the subgroup analysis of the observational studies nor that of the RCTs showed statistical significance between the OAC therapy group and no-OAC group, while the overall pooled estimate indicated that the risk of IS was not statistically different between the OAC group and the no-OAC group, though the upper CI was on the borderline (5 studies, 13,744 participants, OR: 0.41, 95% CI: 0.16 to 1.00, I2=77%, P=0.05) (Figure 2A).
IS or SE
When it came to IS or SE events, 10 studies were pooled for analysis with the M-H random model. Within these 10 studies, OAC therapy was found to be associated with a lower risk only in the subgroup of 8 observational studies (4,894 participants, OR: 0.44, 95% CI: 0.26 to 0.75, I2=57%, P=0.002), while analysis of the 2 RCTs showed no result of statistical significance (304 participants, OR: 0.37, 95% CI: 0.05 to 2.79, I2=81%, P=0.34). To sum up, as shown in Figure 2B, the overall results indicated a better protective effect in the OAC group than in the no-OAC group regarding IS or SE (10 studies, 5,198 participants, OR: 0.42, 95% CI: 0.26 to 0.70, I2=59%, P=0.0008).
All-cause death
A total of 10 studies compared the all-cause mortality between patients who received OAC and those who did not. No statistical significance could be observed in the subgroup analysis of RCTs (2 studies, 304 participants, OR:1.18, 95% CI: 0.60 to 2.33, I2=10%, P=0.63), while the subgroup of observational studies revealed a significantly lower risk of all-cause mortality in the OAC group than the no-OAC group (8 studies, 4,894 participants, OR: 0.49, 95% CI: 0.40 to 0.60, I2=9%, P<0.00001). There was significant heterogeneity between the result of subgroup studies of observational studies and RCTs in terms of all-cause death (I2=83.4%). As was shown in Figure 2C, the overall pooled OR of all-cause mortality was significantly lower for patient receiving OAC therapy than for those avoiding it (10 studies, 5,198 participants, OR: 0.54, 95% CI: 0.41 to 0.70, I2=42%, P<0.00001).
Safety outcomes of OAC therapy vs. no-OAC therapy
ICH recurrence
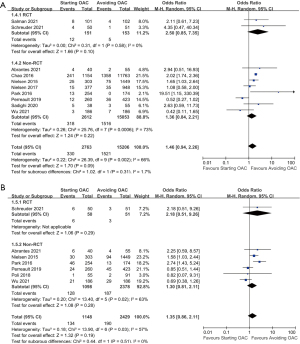
For ICH recurrence, 10 studies were included for analysis. Neither the included 2 RCTs (304 participants, OR: 2.50, 95% CI: 0.85 to 7.35, P=0.10) nor the 8 observational studies (17,665 participants, OR: 1.36, 95% CI: 0.84 to 2.21, P=0.22) revealed statistically significant difference between the OAC and the no-OAC groups. To sum up, the rate of ICH recurrence was not significantly increased in patients receiving OAC compared with those avoiding it (10 studies, 17,969 participants, OR: 1.46, 95% CI: 0.94 to 2.26, I2=66%, P=0.09). The detailed data were shown in Figure 3A.
Major bleeding event
Seven studies provided data that allowed for a comparison of the occurrence of major bleeding events in the OAC group and the no-OAC group. Neither the included RCT, APACHE-AF (101 participants, OR: 2.18, 95% CI: 0.51 to 9.26, P=0.29) nor the remaining 6 observational studies (3,476 participants, OR: 1.30, 95% CI: 0.81 to 2.11, P=0.28) revealed statistically significant difference. The overall results, shown in Figure 3B, indicated no significant increase in the rate of major bleeding events in patients receiving OAC compared to those avoiding it (7 studies, 3,577 participants, OR: 1.35, 95% CI: 0.86 to 2.11, I2=57%, P=0.19).
Publication bias and quality assessment
Publication bias was determined using the funnel plots (Figure 4). Symmetry funnel plots of each outcome measure could be obtained from visual inspection of relevant test results. In addition, the egger’s test was conducted to precisely detect the publication bias and no statistically significant bias was observed (detailed information provided in Figure S1).

Overall, 8 out of 11 (72.7%) studies reported consecutive recruitment. Selection bias was minimized in 2 studies through the random selection of patients in RCTs. Blinding of outcome assessment was performed in these 2 RCTs. The investigators were not blinded to the anticoagulant status in any of the included studies. Attrition bias was found in one study. The risk of a reporting bias was low in all studies. Potential risks of bias are presented in Table S1.
Sensitivity analysis and investigation of heterogeneity
Sensitivity analysis was performed by excluding one study a time. As a result, the pooled effect did not change substantially. Given the moderate to significant heterogeneity in the random effects model assessing all the outcomes, a meta-regression was performed to identify factors that could have caused the heterogeneity. Studies were appraised based on potential factors such as mean age, gender (percentage of female), type of ICH (inclusion of intraparenchymal hemorrhages only), CHA2DS2-VASc score, and type of OACs (VKAs only or VKAs/DOACs). Each of these factors was assessed individually. Consequently, gender was found to be a potential confounding factor that may contribute to the heterogeneity in the evaluation of all-cause death (coefficient =0.969, SE =0.012, t=−2.54, 95% CI: 0.942 to 0.997, P=0.035, adjusted R2=0.93). Detailed information of the meta-regression was presented in Table S2 and Figure S2.
Discussion
For AF patients who survived ICH, whether to prescribe OACs is a dilemma that clinicians usually face, as it is difficult to balance between the risk of thromboembolism and a recurrent hemorrhage. Anticoagulation therapy for AF patients surviving ICH was still a controversial issue and decisions are mainly based on observational studies and expert consensus (2,5,6,22). Therefore, we performed this most comprehensive meta-analysis, the first of this kind to include two most recently published RCTs (11,12), investigating the optimal therapeutic strategy and comparing the effectiveness and safety of starting OAC therapy and avoiding OAC in AF patients with a history of ICH during long-term follow-up. In this systematic review and meta-analysis of 11 studies with more than 18,000 AF patients surviving ICH, we found that starting OAC therapy after ICH was associated with a lower occurrence of IS or SE, and all-cause death than avoiding OAC therapy, without increasing ICH recurrence or major hemorrhage event significantly.
As for effectiveness outcome, our meta-analysis showed that starting OACs after ICH was associated with a significant decrease in the occurrence of IS or SE, and all-cause death, by 58% and 46% respectively compared to avoiding OAC therapy. However, subgroup study of non-RCTs and RCTs showed heterogeneity: starting OAC was only revealed to have a lower risk in the observational studies, while for RCTs, the result had no statistical significance between starting OAC and avoiding OAC in terms of the risk of IS/SE and all-cause death. The included observational studies are highly prone to selection bias and confounding by indication because clinicians may avoid the prescription of OAC in patients with a short life expectancy or at a higher risk of death. Randomization procedure secured well balanced patient and imaging characteristics in RCTs, while in observational studies, people with larger intracerebral hemorrhage volumed were less likely to be prescribed with OAC. What is more, in the retrospective observational studies, some deaths may be attributed to undiagnosed stroke and some minor strokes may have remained undetected, making it possible that the risk of IS was underreported.
When it comes to the safety outcome, the main concern regarding OAC resumption is the recurrence of ICH. Previous research showed that ICH survivors carried a significant risk of recurrent ICH when treated with VKA (23). We found no significant increase in the risk of a recurrent ICH or major bleeding for those receiving OAC, although the point estimate for the pooled OR was 46% and 35% higher than when OAC was avoided. Subgroup analysis showed consistency in the RCT group and the non-RCT group. However, certain limitations of published studies, especially the fact that most of them are observational studies, may have influenced this finding. In particular, the results might be influenced by confounding by indication and selection bias in the observational studies, in which patients at a higher perceived risk of bleeding may have been less likely to be prescribed with OAC, while those with smaller hematomas or a lower risk of bleeding are more likely to receive OAC. Moreover, not all studies provided information on hematoma volume and hematoma location, or if the ICH was a first-time bleed or recurrent hemorrhage. The lack of data on baseline information regarding ICH precluded further evaluation.
The optimal timing of starting anticoagulant treatment after ICH with AF patients was inconclusive. A cohort study suggested that anticoagulant treatment might be initiated 7 to 8 weeks after ICH in ICH survivors with AF to balance benefits from the treatment against the risk of a recurrent bleeding (24). In the studies included in our meta-analysis, the time interval between ICH and the start of the OAC therapy varied from 34 days to 117.5 days. However, such information was not provided in some of the studies, which precluded further evaluation.
Three studies included in our systematic review enrolled only patients who used VKAs as the OACs, while patients in 7 studies were prescribed with DOACs or VKAs as the anticoagulation therapy. Further subgroup analyses to compare the effect of DOACs with that of VKA or to compare the effect of different types of DOACs were not possible because relevant information was missing in several studies. DOACs have been shown to be associated with a significantly lower risk of hemorrhagic complications including ICH compared to VKA (1,25,26). Apixaban significantly reduces the risk of stroke and SE compared to aspirin without increasing the risk of ICH (27). Therefore, DOACs could be a better option for AF patients surviving ICH. One of the RCTs included in our meta-analysis, Apixaban Versus Antiplatelet Drugs or no Antithrombotic Drugs After Anticoagulation-Associated Intracerebral Hemorrhage in Patients with Atrial Fibrillation (APACHE-AF) pilot study (11), addressed stroke prevention with apixaban in ICH survivors with AF. However, the result showed that the risks of IS or recurrent ICH were similar in participants allocated to apixaban and in those assigned to avoiding anticoagulation. This study had a small sample size of 101 patients, and as a result, event rates of the outcome had a wide 95% CI and the assessment of the efficacy and safety of OACs was inconclusive. This highlights the importance of meticulous interpretation of the aggregate results and the subgroup results of RCTs.
Our study shed light on the limitations of existing studies focusing on OAC use in AF patients with ICH. First, most of the studies were observational studies lacking randomization, which might affect the reliability of the results, although there were two completed open-label RCTs. Therefore, this meta-analysis was subject to the limitations of the non-blinded, mostly retrospective observational design of the included studies. Secondly, the two RCTs included in our meta-analysis, which has a low risk of selection bias and little indication confounding, nevertheless, also had limitations in that the sample sizes were small and as a result, the assessment of the efficacy and safety of OACs was inconclusive. Thirdly, there existed heterogeneity among the included studies, including considerable heterogeneity between RCTs and non-RCTs in terms of evaluating all-cause mortality. Meta-regression found that gender might be a confounding factor attributing to the heterogeneity in the evaluation of all-cause death. Besides, wide variations in the demographics of enrolled patients and study designs, including the different follow-up durations in the individual studies, time interval before starting OAC, or the existence of co-morbidities, might have contributed to the heterogeneity. Fourthly, the selection criteria may vary across individual studies as some included only patients with intraparenchymal hemorrhage while others widened their coverage to include also subdural and subarachnoid hemorrhages. Lastly, information on blood pressure control, which was a known risk factor of ICH recurrence and might affect the outcome (28), was not provided in all the included studies.
Considering the heterogeneity of the existing studies, more high-grade evidences are needed to solve the clinical dilemma, and there are five ongoing pivotal RCTs: STATICH (NCT03186729), A3ICH (NCT03243175), ASPIRE (NCT03907046), ENRICH-AF (NCT03950076), and PRESTIGE-AF (NCT03996772). It is our hope that these will provide more substantial evidences in the field of anticoagulation in AF patients with a history of ICH (Table S3). Pooled analysis is probably required when more RCTs are completed in the future to resolve this therapeutic dilemma.
Conclusions
This meta-analysis of OAC therapy in AF patients with a history of ICH suggested that starting OAC therapy after ICH was associated with lower rates of IS, IS or SE, and all-cause mortality than avoiding OAC, without increasing ICH recurrence or major hemorrhage events significantly. Considering the heterogeneity between the results of observational studies and RCTs, as well as the limited number and small sample size of the RCTs, high-grade evidences, including more RCTs with larger sample sizes, are needed to better inform clinical decisions regarding anticoagulation therapy in AF patients with ICH.
Acknowledgments
Sincere gratitude goes to Professor Ling Yue, who contributed to editing the language of this paper.
Funding: This study was supported by grants from the Scientific Research Seed Fund of Peking University First Hospital (No. 2020SF22) and Peking University Baidu Fund (No. 2019BD019).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-582/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-582/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. [Crossref] [PubMed]
- Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021;23:1612-76. [Crossref] [PubMed]
- Alonso A, Bengtson LG, MacLehose RF, et al. Intracranial hemorrhage mortality in atrial fibrillation patients treated with dabigatran or warfarin. Stroke 2014;45:2286-91. [Crossref] [PubMed]
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62. [Crossref] [PubMed]
- Rivera-Caravaca JM, Esteve-Pastor MA, Camelo-Castillo A, et al. Treatment strategies for patients with atrial fibrillation and anticoagulant-associated intracranial hemorrhage: an overview of the pharmacotherapy. Expert Opin Pharmacother 2020;21:1867-81. [Crossref] [PubMed]
- Nguyen NY, Frishman WH. Restarting Oral Anticoagulation in Patients With Atrial Fibrillation After an Intracranial Hemorrhage. Cardiol Rev 2020;28:190-6. [Crossref] [PubMed]
- Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:2032-60. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol 2021;134:178-89. [Crossref] [PubMed]
- Schulman S, Angerås U, Bergqvist D, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010;8:202-4. [Crossref] [PubMed]
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. Chinester: Wiley; 2008.
- Schreuder FHBM, van Nieuwenhuizen KM, Hofmeijer J, et al. Apixaban versus no anticoagulation after anticoagulation-associated intracerebral haemorrhage in patients with atrial fibrillation in the Netherlands (APACHE-AF): a randomised, open-label, phase 2 trial. Lancet Neurol 2021;20:907-16. [Crossref] [PubMed]
- SoSTART Collaboration. Effects of oral anticoagulation for atrial fibrillation after spontaneous intracranial haemorrhage in the UK: a randomised, open-label, assessor-masked, pilot-phase, non-inferiority trial. Lancet Neurol 2021;20:842-53. [Crossref] [PubMed]
- Nielsen PB, Larsen TB, Skjøth F, et al. Outcomes Associated With Resuming Warfarin Treatment After Hemorrhagic Stroke or Traumatic Intracranial Hemorrhage in Patients With Atrial Fibrillation. JAMA Intern Med 2017;177:563-70. [Crossref] [PubMed]
- Poli L, Grassi M, Zedde M, et al. Anticoagulants Resumption after Warfarin-Related Intracerebral Haemorrhage: The Multicenter Study on Cerebral Hemorrhage in Italy (MUCH-Italy). Thromb Haemost 2018;118:572-80. [Crossref] [PubMed]
- Abrantes CS, Pintalhão M, Tavares S, et al. Anticoagulation after intracerebral hemorrhage in patients with atrial fibrillation: between Scylla and Charybdis. Neurol Sci 2022;43:2441-8. [Crossref] [PubMed]
- Chao TF, Liu CJ, Liao JN, et al. Use of Oral Anticoagulants for Stroke Prevention in Patients With Atrial Fibrillation Who Have a History of Intracranial Hemorrhage. Circulation 2016;133:1540-7. [Crossref] [PubMed]
- Nielsen PB, Larsen TB, Skjøth F, et al. Restarting Anticoagulant Treatment After Intracranial Hemorrhage in Patients With Atrial Fibrillation and the Impact on Recurrent Stroke, Mortality, and Bleeding: A Nationwide Cohort Study. Circulation 2015;132:517-25. [Crossref] [PubMed]
- Park YA, Uhm JS, Pak HN, et al. Anticoagulation therapy in atrial fibrillation after intracranial hemorrhage. Heart Rhythm 2016;13:1794-802. [Crossref] [PubMed]
- Perreault S, Côté R, White-Guay B, et al. Anticoagulants in Older Patients with Nonvalvular Atrial Fibrillation after Intracranial Hemorrhage. J Stroke 2019;21:195-206. [Crossref] [PubMed]
- Sadighi A, Wasko L, DiCristina H, et al. Long-term outcome of resuming anticoagulation after anticoagulation-associated intracerebral hemorrhage. eNeurologicalSci 2020;18:100222. [Crossref] [PubMed]
- Wu VC, Huang YC, Chen SW, et al. Resuming anticoagulation in patients with atrial fibrillation experiencing intracranial hemorrhage. Medicine (Baltimore) 2021;100:e26945. [Crossref] [PubMed]
- da Silva IRF, Frontera JA. Resumption of Anticoagulation After Intracranial Hemorrhage. Curr Treat Options Neurol 2017;19:39. [Crossref] [PubMed]
- Poli D, Antonucci E, Dentali F, et al. Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014;82:1020-6. [Crossref] [PubMed]
- Pennlert J, Overholser R, Asplund K, et al. Optimal Timing of Anticoagulant Treatment After Intracerebral Hemorrhage in Patients With Atrial Fibrillation. Stroke 2017;48:314-20. [Crossref] [PubMed]
- Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur Stroke J 2019;4:198-223. [Crossref] [PubMed]
- Chatterjee S, Sardar P, Biondi-Zoccai G, et al. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013;70:1486-90. [Crossref] [PubMed]
- Peterson BE, Al-Khatib SM, Granger CB. Apixaban to prevent stroke in patients with atrial fibrillation: a review. Ther Adv Cardiovasc Dis 2017;11:91-104. [Crossref] [PubMed]
- Biffi A, Anderson CD, Battey TW, et al. Association Between Blood Pressure Control and Risk of Recurrent Intracerebral Hemorrhage. JAMA 2015;314:904-12. [Crossref] [PubMed]



