
The efficacy and safety of the Xuesaitong soft capsule in the treatment of patients with ischemic stroke: systematic review and meta-analysis
Introduction
Ischemic stroke is 1 of the 3 leading causes of death, and the main cause of severe disability in developed countries (1). Approximately 15 million patients suffer from ischemic stroke per year worldwide, of whom, 1/3 die and 1/3 suffer from a permanent disability, which places serious economic burdens on families, communities, and countries (2). The brain damage caused by ischemic stroke is the results of the interaction of complex pathophysiological processes, such as inflammation, apoptosis, oxidative stress, and excitotoxicity (3). The current treatments for patients with ischemic stroke include aspirin and thrombolytic therapy (4). Other effective drugs, such as the Xuesaitong soft capsule, can be used in addition to these 2 methods.
The Xuesaitong soft capsule has various effects, such as improving hemorheology and hemodynamics, dilating cerebral blood vessels, anti-thrombotic effects, and inhibiting platelet aggregation (5). The main ingredients of the Xuesaitong soft capsule are panax notoginseng saponins (PNSs). In the 1980s, Kunming Pharmaceutical used PNSs for the first time in clinical applications, developed and named a product, “Xuesaitong”, and launched that product in Vietnam, Cambodia, Myanmar, and Tanzania. The main active ingredient of PNSs was panax notoginseng. Panax notoginseng contains many types of monomeric saponins, among which the levels of notoginsenoside (R1) and ginsenosides (Rb and Rg) are very high (6). According to previous research (7), PNSs have anti-cerebral ischemia, anti-thrombosis, and other pharmacological effects, lower blood lipids, and improve cerebral blood circulation. PNSs are widely used in the treatment of diseases of the cardiovascular system, such as cerebral infarction (8). As a new type of preparation (9), the Xuesaitong soft capsule cannot only be absorbed faster in the digestive tract, but also has good bioavailability.
With the further development of traditional Chinese medicine (TCM) in clinical applications, a new dosage form that has convenient storage and high bioavailability has been developed. As a new type of preparation, the Xuesaitong soft capsule overcomes the disadvantages, such as the low bioavailability, of oral preparations (e.g., hard gelatin capsules and troches) (9). Xuesaitong is one of the most commonly used medicines for treating ischemic stroke in China. However, compared to the conventional therapy, the effectiveness and safety of Xuesaitong for ischemic stroke needs to be systematically reviewed. This is the first meta-analysis to evaluate the clinical efficacy and safety of the Xuesaitong soft capsule in treating patients with ischemic stroke. Our findings provide a reference for drug selection in patients with ischemic stroke. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-748/rc).
Methods
Sources of information
We searched several databases, such as the China National Knowledge Infrastructure, Wanfang, VIP, PubMed, Embase, and Web of Science databases, and collected all the randomized controlled trials (RCTs) on the use of Xuesaitong soft capsule in patients with ischemic stroke. All the databases were searched using the following keywords: “Xuesaitong”, “panax notoginsenoside”, “panax notoginsenosides”, “soft capsule”, “cerebral ischemic stroke”, “apoplexy”, “stroke”, “ischemic cerebrovascular disease”, “ischemic cerebral diseases”, “brain infarction”, “cerebral infarction”, “infarct of brain”, “cerebral ischemia”.
Inclusion criteria
Articles were included in the meta-analysis if they met the PICOS principles as following: (I) P (Population) the study participants included patients diagnosed with ischemic stroke, regardless of their nationality, gender, and race; (II) S (Study design) the article concerned a RCT; (III) I (Intervention) C (Comparison) the test group received a Xuesaitong soft capsule treatment or a conventional treatment plus the Xuesaitong soft capsule, while the control group received a conventional treatment or other drug treatment; and (IV) O (Outcome) the outcomes included the total effective rate, Clinical Severity Score (CSS scores), the Barthel index (BI) score, adverse reactions, plasma viscosity, whole-blood viscosity at high and low shear rates, fibrinogen, hematocrit, the effect on TCM single symptoms or signs (e.g., crooked mouth and tongue, and dizziness), and the therapeutic efficacy of TCM syndrome.
Exclusion criteria
Articles were excluded from the meta-analysis if they met any the following exclusion criteria: (I) the study had no control group; (II) the raw data were incomplete; (III) the article concerned a letter, case report, or review; (IV) randomness was not mentioned in the article; and/or (V) the study design was not rigorous (e.g., non-uniform criterion for the result judgment or unclear or incomplete sample data).
Selection and evaluation of the articles
In this study, 2 researchers independently read the titles and abstracts to screen out the irrelevant articles and read the full text to evaluate whether each article met the inclusion criteria. If the researchers’ opinions differed, the issue was discussed with or determined by a 3rd researcher.
The following information was extracted from the articles: the first author, year of publication, number of study participants, mean age, intervention, dosage of the Xuesaitong soft capsule, treatment time, and the general disease history of the participants.
The Cochrane Collaboration’s risk of bias tool was used for the article evaluation. The evaluation items examined the following 7 aspects: sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective outcome reporting (reporting bias); and other sources of bias (other bias). Each aspect was ranked as either “low risk”, “unclear risk”, or “high risk”.
Statistical analysis
STATA 15.0 software was used to analyze the data. The continuous variables were measured by the mean difference (MD) in terms of the average value and standard deviation. The dichotomous outcomes were measured by the odds ratio (OR), and were calculated with the 95% confidence interval (95% CI). The heterogeneity analysis was conducted by calculating the χ2 and I2. The combined effect results are presented in forest maps. The test level was α=0.1, such that a P value ≤0.1 or an I2 value ≥50%, indicated that there was heterogeneity among the included articles, in which case, the random-effects model was selected. Conversely, a P value >0.1 or an I2 value <50% indicated that there was little heterogeneity, in which case, the fixed-effects model was selected. To avoid the influence of heterogeneity among the included studies, the subgroup analysis was carried out according to possible heterogeneity factors. P <0.05 was considered statistically significant in all the analyses.
Results
Study identification and characteristics
A total of 914 studies were retrieved using the above-mentioned search strategy. A total of 1,214 patients were included in the Xuesaitong treatment group and 764 patients were included in the control group. And the control group included patients who had undergone conventional treatment, comprehensive treatment and Ginkgo biloba treatment. After reading the titles and abstracts, the following articles were excluded: duplicate articles (n=483), non-clinical research studies (n=21), and non-RCTs (n=196). After which, 214 articles were preliminarily included in the study. After reading the full text of all the 214 articles, 97 articles were removed because records excluded based on information provided by titles, abstracts and author’s unit, 58 articles excluded reports were not retrieved. 32 articles because the sample size was less than 70, and 10 because the data were incomplete. Ultimately, 17 articles were included in the meta-analysis (see Figure 1).
The 17 included articles comprised a total of 1,942 patients, including 1,254 in the test group and 688 in the control group. The age of patients ranged from 37 to 80 years. The treatment time ranged from 2 weeks to 24 weeks. All the included articles concerned RCTs. The basic information of the included articles (10-26) is shown in Table 1.
Table 1
| Included articles | Patients (n) | Age (years), range or mean ± SD | Interventions | Dose (Xuesaitong) | Treatment time (weeks) | History of comorbidity |
|---|---|---|---|---|---|---|
| Wu et al. [2011] (10) | ||||||
| Test | 354 | 62.53±8.85 | Xuesaitong soft capsule | 2 tabletsa | 4 | No |
| Control | 118 | 61.87±8.86 | Ginkgo biloba capsule | – | – | – |
| Su et al. [2005] (11) | ||||||
| Test | 108 | 44–75 (52±7) | Xuesaitong soft capsule | 3 tabletsb | 8 | Hypertension, diabetes, coronary heart disease, basal ganglia infarction, cerebral lobe infarction |
| Control | 96 | – | Comprehensive medical | – | – | Brain stem infarction, cerebellar infarction |
| Liu et al. [2005] (12) | ||||||
| Test | 100 | 40–76 | Xuesaitong soft capsule | 120 mgb | 2 | Hypertension, diabetes, hyperlipidemia |
| Control | 50 | 45–72 | Low molecular dextran | Hypertension, diabetes, hyperlipidemia | ||
| Ding et al. [2015] (13) | ||||||
| Test | 60 | 45–74 | Xuesaitong soft capsule | 100–200 mga | 4 | No |
| Control | 60 | 45–72 | Conventional treatment | – | – | – |
| Li et al. [2012] (14) | ||||||
| Test | 83 | 39–75 | Xuesaitong soft capsule | 2 tabletsa | 4 | No |
| Control | 27 | 48–75 | Ginkgo biloba capsule | – | – | – |
| Li et al. [2010] (15) | ||||||
| Test | 84 | 39–76 | Xuesaitong soft capsule | 2 tabletsa | 4 | No |
| Control | 28 | – | Ginkgo biloba capsule | – | – | – |
| Wang et al. [2008] (16) | ||||||
| Test | 54 | 45–74 | Xuesaitong soft capsule | 2 tabletsb | 4 | No |
| Control | 54 | 42–73 | Xinnaoshutong capsule | – | – | – |
| Jia et al. [2017] (17) | ||||||
| Test | 50 | 61–76 | Xuesaitong soft capsule | 2 tabletsa | 24 | No |
| Control | 50 | 60–75 | Conventional treatment | – | – | – |
| Wang et al. [2017] (18) | ||||||
| Test | 43 | 45–75 | Xuesaitong soft capsule | 2 tabletsa | 8 | No |
| Control | 43 | 43–77 | Foundation treatment | – | – | – |
| Mi et al. [2009] (19) | ||||||
| Test | 45 | 52–76 | Xuesaitong soft capsule | 2 tabletsa | 4 | No |
| Control | 40 | 53–78 | Comprehensive medical | – | – | – |
| Guo et al. [2005] (20) | ||||||
| Test | 40 | 52–80 | Xuesaitong soft capsule | 2 tabletsa | 12 | No |
| Control | 40 | 48–77 | Nimodipine | – | – | – |
| Zhao et al. [2004] (21) | ||||||
| Test | 40 | 39–72 | Xuesaitong soft capsule | 2 tabletsb | 4 | High TC, high TG, high TC and TG |
| Control | 40 | 38–70 | Zhibituo | – | – | High TC, high TG, high TC and TG |
| Li et al. [2002] (22) | ||||||
| Test | 35 | 49–71 | Xuesaitong soft capsule | 120 mgb | 4 | No |
| Control | 30 | 48–73 | Comprehensive medical | – | – | – |
| Liu et al. [2017] (23) | ||||||
| Test | 53 | 61–78 | Xuesaitong soft capsule | 100 mga | 24 | No |
| Control | 53 | 60–79 | Conventional treatment | – | – | – |
| Yang et al. [2006] (26) | ||||||
| Test | 33 | 45–70 | Xuesaitong soft capsule | 2 tabletsa | 14 | No |
| Control | 33 | 50–76 | Nimodipine | – | – | – |
| Lv et al. [2011] (24) | ||||||
| Test | 27 | 35–77 | Xuesaitong soft capsule | 3 tabletb | 12 | No |
| Control | 27 | – | Aspirin | – | – | – |
| Gao et al. [2012] (25) | ||||||
| Test | 45 | 39–76 | Xuesaitong soft capsule | 2 tabletsa | 4 | No |
| Control | 15 | – | Ginkgo biloba capsule | – | – | – |
a, 3 times a day; b, twice a day. SD, standard deviation; TC, total cholesterol; TG, triglyceride.
Study quality
Figures 2,3 show an example of the risk bias assessment provided by the Cochrane Systematic Review Manual. Different colors (i.e., green, red, and yellow) are used to indicate different types of bias (i.e., “low-risk bias”, “high-risk bias”, and “unclear bias”, respectively). Five are high-quality low-risk articles and the rest are unknown or high-risk articles. In general, the quality of study reporting was relatively low, suggesting an overall high risk of bias in the included studies.
Pooled effective rate
Of the 17 included articles, 4 (11,16,20,26) reported the total effective rate of the Xuesaitong soft capsule in the treatment of patients with ischemic stroke. A pooled analysis was performed with the effective rate as the binary variable. The meta-analysis results showed that the pooled effective rate of using the Xuesaitong soft capsule (test group) was significantly higher than that of conventional or other drug treatments (the control group) (OR =3.24, 95% CI: 2.21, 4.76, Z=6.01, P<0.001]. The subgroup analysis revealed that the total effective rate of using the Xuesaitong soft capsule was significantly higher in the test group than the control group at 4, 8, and 12 weeks (4 weeks OR =2.72, 95% CI: 1.24, 5.97, Z=2.50, P=0.013; 8 weeks OR =5.38, 95% CI: 2.95, 9.83, Z=5.48, P<0.001; 12 weeks OR =1.97, 95% CI: 1.01, 3.84, Z=2.00, P=0.046). There was no significant heterogeneity among the included articles (I2=44.0%, P=0.147; see Figure 4).

CSS score
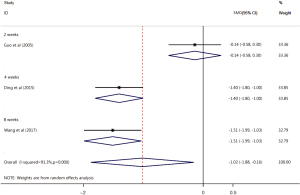
Of the 17 included articles, 3 examined CSS score improvement after treatment with the Xuesaitong soft capsule (13,18,20). The CSS scores of patients with ischemic stroke in the test group was significantly lower than those of patients in the control group (SMD =–1.02, 95% CI: –1.88, –0.16, Z=2.32, P=0.02). The heterogeneity among the included articles was significant (I2=91.3%, P=0.000). The subgroup analysis revealed no significant differences in the CSS scores between the Xuesaitong soft capsule treatment test group and the conventional treatment control group at 2 weeks (standardized mean difference (SMD) =–0.14, 95% CI: –0.58, 0.30, Z=2.32, P=0.536). However, the Xuesaitong soft capsule improved the CSS scores of the test group more than those of the control group at 4 and 8 weeks, and the difference was statistically significant (4 weeks SMD =–1.40, 95% CI: –1.80, –1.00, Z=6.88, P<0.001; 8 weeks SMD =–1.51, 95% CI: –1.99, –1.03, Z=6.17, P<0.001; see Figure 5).
BI score
Of the 17 included articles, 4 (12,20,24,26) showed that the BI score of the Xuesaitong soft capsule treatment test group was significantly higher than that of the conventional drug treatment control group (SMD =1.12, 95% CI: 0.83, 1.42, Z=7.47, P<0.001). The heterogeneity among the included articles was significant (I2=64.7%, P=0.009). The subgroup analysis showed that the BI score of the test group was significantly higher than that of the control group for patients with ischemic stroke at 2, 4, and 12 weeks (2 weeks, SMD =0.69, 95% CI: 0.35, 1.04, Z=3.91, P<0.001; 4 weeks SMD =1.06, 95% CI: 0.69, 1.42, Z=5.72, P<0.001; 12 weeks SMD =1.30, 95% CI: 0.84, 1.77, Z=5.46, P<0.001; see Figure 6).

Plasma viscosity
Of the 17 included articles, 4 (13,19,22,23) reported an improvement in the plasma viscosity of patients treated with the Xuesaitong soft capsule. The heterogeneity among the included articles was significant (I2=97.6%, P<0.001). The random-effects model showed that the plasma viscosity of the test group treated with the Xuesaitong soft capsule was significantly more improved than that of the control group (MD =–4.66, 95% CI: –7.13, –2.20, P<0.001; see Table 2).
Table 2
| Index | Number of included studies | Patients | Heterogeneity test | Combined model | MD (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| I2 | P value | ||||||
| Plasma viscosity | 4 | 376 | 97.6% | <0.001 | Random-effects model | –4.66 (–7.13, –2.20) | <0.001 |
| Whole-blood viscosity at low shear rates | 4 | 376 | 0% | 0.601 | Fixed-effects model | –0.73 (–0.94, –0.52) | <0.001 |
| Whole-blood viscosity at high shear rates | 4 | 376 | 85.9% | <0.001 | Random-effects model | –1.64 (–2.28, –1.00) | <0.001 |
| Fibrinogen level | 3 | 270 | 0% | 0.839 | Fixed-effects model | –1.58 (–1.86, –1.31) | <0.001 |
| Hematocrit | 3 | 270 | 0% | 0.887 | Fixed-effects model | –2.13 (–2.43, –1.83) | <0.001 |
CI, confidence interval; MD, mean difference.
Whole-blood viscosity at low and high shear rates
Of the 17 included articles, 4 (13,19,22,23) reported that the patients treated with the Xuesaitong soft capsule had improved whole-blood viscosity at low and high shear rates. There was no significant heterogeneity among the included articles examining whole-blood viscosity at low shear rates (I2=0%, P=0.601). The fixed-effects model showed that compared to the conventional treatment, the Xuesaitong soft capsule treatment significantly reduced the level of whole-blood viscosity at low shear rates (MD =–0.73, 95% CI: –0.94, –0.52, P<0.001). There was significant heterogeneity among the included articles examining whole-blood viscosity at high shear rates (I2=85.9%, P<0.001). The random-effects model showed that compared to the conventional treatment, the Xuesaitong soft capsule treatment significantly reduced the level of whole-blood viscosity at high shear rates (MD =–1.64, 95% CI: –2.28, –1.00, P<0.001; see Table 2).
Fibrinogen level
Of the 17 included articles, 3 (13,19,22) examined the patients’ fibrinogen levels and were analyzed by the fixed-effects model. The heterogeneity test analysis showed that there was no heterogeneity among the included articles (I2=0%, P=0.839). The meta-analysis results showed that the Xuesaitong soft capsule treatment significantly reduced the patients’ fibrinogen levels compared to the conventional treatment, and the difference was statistically significant (MD =–1.58, 95% CI: –1.86, –1.31, P<0.001; see Table 2).
Hematocrit
Of the 17 included articles, 3 (13,19,22) examined hematocrit. The results of the meta-analysis showed that the Xuesaitong soft capsule treatment significantly reduced hematocrit compared to the conventional treatment (MD =–2.13, 95% CI: –2.43, –1.83, P<0.001). The heterogeneity test analysis showed that there was no heterogeneity among the included articles (I2=0%, P=0.887; see Table 2).
The effect on TCM single symptoms or signs
Of the 17 included articles, 2 (10,15) reported the recovery rate, efficacy, and the pooled effective rate of TCM single symptoms or signs (e.g., crooked mouth and tongue, and dizziness). The pooled effective rate for TCM single symptoms or signs in the test group was better than that of the control group, and the difference was statistically significant (P<0.05; see Table 3).
Table 3
| Symptoms | Studies | Group | Recovery rate (%) | Markedly effective rate (%) | Pooled effect (%) | P value |
|---|---|---|---|---|---|---|
| Crooked mouth and tongue | Wu [2011] (10) | Control group (n=101) | 16.83 | 17.82 | 45.54 | 0.01 |
| Test group (n=328) | 32.01 | 39.02 | 59.45 | |||
| Li [2010] (15) | Control group (n=25) | 16.0 | 16.0 | 32.0 | <0.05 | |
| Test group (n=81) | 33.3 | 35.8 | 42.0 | |||
| Dizziness | Wu [2011] (10) | Control group (n=83) | 59.04 | 62.65 | 73.49 | 0.027 |
| Test group (n=262) | 68.70 | 73.66 | 84.35 | |||
| Li [2010] (15) | Control group (n=20) | 60.00 | 60.00 | 60.00 | <0.05 | |
| Test group (n=62) | 56.50 | 61.30 | 67.70 |
TCM, traditional Chinese medicine.
The therapeutic efficacy of TCM syndrome
Of the 17 included articles, 2 (10,25) reported the recovery rate, efficacy, and total effective rate of TCM syndrome. The total effective rate of the TCM syndrome in the test group was better than that of the control group, but only 1 article (27) reported that the differences were statistically significant (P<0.05; see Table 4).
Table 4
Adverse reactions
Of the 17 included articles, 5 (10,13-15,24) reported on the occurrence of adverse reactions; 12 articles did not report any obvious adverse reactions. The heterogeneity test analysis showed that there was heterogeneity among the included articles (I2=0.0%, P=0.480), and the fixed-effects model was used for the combined analysis. The meta-analysis results showed that there was no significant difference in the adverse reactions between the test group and the control group (OR =2.52, 95% CI: 0.87, 7.32, P=0.088; see Figure 7).

Discussion
The meta-analysis results showed that compared to conventional or other drug treatments, the Xuesaitong soft capsule treatment has significant clinical benefits for patients with ischemic stroke. In addition to improving the TCM symptoms of patients with ischemic stroke, such as crooked mouth and tongue, and dizziness, the long-term use of the Xuesaitong soft capsule is also beneficial to the recovery of patients’ nerve function and quality of life. Previous meta-analyses have mainly evaluated the effects of Xuesaitong injections on patients with ischemic stroke.
The efficacy assessment results showed that the Xuesaitong soft capsule had a significantly higher total effective rate for patients with ischemic stroke than conventional or other drug treatments. Similar to previous research results, a clinical study of 204 patients with acute cerebral infarction (27) that used internationally recognized neurological defect score changes and clinical efficacy as the observation indicators showed that the total efficacy of patients with acute cerebral infarction in the Xuesaitong treatment group after 8 weeks of treatment was significantly higher than that of the conventional treatment group. For patients with acute cerebral infarction, Xuesaitong improves clinical efficacy, possibly by inhibiting the calcium load of brain cells (28), stabilizing cell membranes, protecting reperfusion injury caused by ischemia, reducing platelet aggregation, and reducing brain cell oxygen consumption (29).
When the CSS scores in the articles were aggregated and analyzed, the results showed that Xuesaitong soft capsule treatment improved patients’ CSS scores more than conventional or other drug treatments. Consistent with the results of Chang et al. (30), the CSS scores of the Xuesaitong soft capsule test group were lower than those of the control group. These findings suggest that the Xuesaitong soft capsule effectively protects the nerve function of patients with ischemic stroke and promotes the recovery of nerve function damage. It may be that the multifaceted cardio-cerebrovascular pharmacological effects of the Xuesaitong soft capsule treatment improves the CSS score of patients by promoting cerebral blood circulation, increasing cerebral blood flow, and reducing blood viscosity. Additionally, the Xuesaitong soft capsule treatment reduces whole-blood viscosity and hematocrit and improves the oxygen tolerance of brain cells, so that the delayed injury and neurological function can be repaired (31).
The BI is a widely used personal daily living ability scale with good reliability and validity worldwide (32). The statistical analysis showed that the Xuesaitong soft capsule treatment is significantly better than conventional or other drug treatments at improving patients’ BI scores. Liu et al. (12) found that there was no statistically significant difference in the improvement of patients’ BI scores between the low molecular dextran and the Xuesaitong soft capsule groups at 14 days; however, at 90 days, the Xuesaitong soft capsule was found to increase the BI scores compared to low molecular dextran. Thus, if the treatment time is longer than 14 days, the Xuesaitong soft capsule had a better effect on patients’ BI scores. Our results were largely similar to those of previous studies, but we did not find that the longer the treatment time for the Xuesaitong soft capsule, the better the improvement in BI scores. This inconsistency in the results may have been caused by the unstable results of the study conducted by Liu et al., for which the number of subjects was small.
In modern TCM research, cerebral infarction is a blood stasis syndrome (33). It is generally thought that systemic or local blood circulation is not smooth because of a change in blood rheology or an increase in blood viscosity, which leads to a physiological function disorder in patients with cerebral infarction (34). Modern pharmacological studies have found that the Xuesaitong soft capsule reduces blood fibrinogen and hematocrit. Additionally, it has also been shown to reduce blood viscosity, inhibit platelet adhesion, reduce platelet aggregation and surface activity, improve microcirculation, and expand blood vessels, thereby further increasing the blood flow of patients’ brain and other organs (35). In terms of hemorheology, this study was consistent with pharmacological studies showing that compared to conventional or other drugs, the Xuesaitong soft capsule can significantly reduce plasma viscosity, whole-blood viscosity at high and low shear rates, fibrinogen, and hematocrit. A clinical study of 120 patients with cerebral infarction showed that the Xuesaitong soft capsule significantly improved the hemorheology indicators of cerebral infarction patients compared to conventional treatment (13).
Wu et al. analyzed the effect of treatments on TCM single symptoms or signs, and showed that the total effective rate of the Xuesaitong soft capsule treatment was 83.19%, and the total effective rate of the Yinxingye capsule treatment was 68.7% (10). Similarly, we found that the total effective rate of TCM symptoms (e.g., crooked mouth and tongue, and dizziness) in the test group was better than that of the control group, and the difference was statistically significant (P<0.05); Gao et al. showed that the total effective rate of the Xuesaitong soft capsule treatment was 97.78%, and the total effective rate of the Yinxingye capsule treatment was 73.33%, and the effective rate of the test group was significantly better than that of the control group (P<0.05) (25). Li et al. showed that the Xuesaitong soft capsule significantly increased the total effective rate of TCM symptoms (e.g., crooked mouth and tongue, and dizziness) compared to the Yinxingye capsule (P<0.05) (14). Due to a lack of articles, we were unable to conduct a more detailed statistical analysis. Thus, the effect of the Xuesaitong soft capsule on TCM syndrome requires further research and exploration.
In terms of safety, the adverse reactions of the Xuesaitong soft capsule include mild gastrointestinal damage, but the incidence is low. This study showed that there was no significant difference in the adverse reactions between the Xuesaitong soft capsule treatment group and the conventional or other drug treatment group. In a clinical observation study involving 1,189 patients with 13 disease types at 16 hospitals, and 7 departments in Baoding, Beijing, Chongqing, Shanghai, Kunming and other cities showed, no adverse reactions were observed in patients after taking the Xuesaitong soft capsule (36). This is largely consistent with our research results. Thus, this product appears to be relatively safe and can be taken regularly in accordance with the prescription.
This meta-analysis had some limitations. First, the Xuesaitong soft capsule is a TCM that is rarely used abroad. Thus, the included articles were Chinese, which may have caused a publication bias. Second, as the Xuesaitong soft capsule is a relatively new preparation, very few studies have been conducted on it, and the quality of the included articles was generally low. Additionally, the blind method and allocation concealment were not adopted by some of the included studies, which may have affected the research results. Due to the limited number of articles, subgroup analysis by control group is not supported. Although a random-effects model was used to control for statistical heterogeneity, clinical heterogeneity may still exist. Thus, high-quality studies with multi-center and large sample sizes are needed for further verification. However, the articles included in this study had relatively uniform clinical efficacy evaluation standards, and the baseline characteristics of the study subjects were basically consistent; thus, the results of this study are relatively accurate and reliable.
Conclusions
In summary, compared to conventional or other drug treatments, the Xuesaitong soft capsule treatment was beneficial in improving patients’ TCM symptoms (e.g., crooked mouth and tongue, and dizziness) and various indicators. The long-term use of the Xuesaitong soft capsule aids the recovery of nerve function and the quality of life of patients with ischemic stroke. Furthermore, Xuesaitong soft capsule may be a safe and effective drug for the treatment of ischemic stroke. And large-scale randomized clinical trials are needed to further confirm our findings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-748/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-748/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381:1995-2008. [Crossref] [PubMed]
- Wicha P, Tocharus J, Janyou A, et al. Hexahydrocurcumin protects against cerebral ischemia/reperfusion injury, attenuates inflammation, and improves antioxidant defenses in a rat stroke model. PLoS One 2017;12:e0189211. [Crossref] [PubMed]
- Liu HY, Fang W. Advances in the pathogenesis of ischemic stroke. China Modern Doctor 2010;48:11-2.
- Jiang YY, Zeng YP, Wu B. Progress in clinical research of cerebrovascular disease. Chinese Journal of Modern Neuropathy 2018;18:89-94.
- Jiang L, Yang L, Zhong J. Clinical research progress of Xuesaitong soft capsule. Chinese Journal of Ethnomedicine and Ethnopharmacy 2016;25:55-7.
- Su J, Zhu WQ. The contents of notoginseng R1, ginsenoside Rb1 and ginsenoside Rg1 in Xuesaitong tablets were determined by RP-HPLC gradient elution method. Chinese Journal of Pharmaceutical Analysis 2005;25:212-4.
- Gan Y, Xu HB. sun XB. Research progress on pharmacological action of total saponins of Panax notoginseng. Lishizhen Medicine and Materia Medica Research 2007;140:1251-2.
- Duan YH, Wu M. Progress in pharmacological research and clinical application of total saponins from Panax notoginseng. Information on Traditional Chinese Medicine 2014;31:108-10.
- Li N, Wang C, Ding J. Xuesaitong soft capsule and its preparation process. CN1228960[P]. 1999.
- Wu Q, Pu J, Wang Z, et al. Clinical observation of Xuesaitong soft capsule in treating stagnation syndrome of meridian convalescence in apoplexy. Chinese Journal of Integrative Medicine on Cardio-Cerebrovascular Disease 2011;9:952-3.
- Su JX, Sun YQ, Sun LJ, et al. Therapeutic effect of Xuesaitong soft capsule on 108 cases of acute cerebral infarction. Shandong Medical Journal 2005;45:3.
- Liu Y, Ge X. Observation on curative effect of Xuesaitong soft capsule in treating 100 cases of acute cerebral infarction. Yunnan Journal of Traditional Chinese Medicine and Materia Medica 2005;26:51.
- Ding X, Zhang X. Clinical experience of xuesaitong soft capsule in treating cerebral infarction. Medical Information 2015;72.
- Li S, Li M, Li W, et al. Effect of Xuesaitong soft capsule on improving self-care ability of patients with cerebral infarction. Chinese Pharmacy 2012;23:4552-3.
- Li S, Hu J, Zhang S. Clinical observation on the safety and effectiveness of Xuesaitong soft capsule in the treatment of middle meridian convalescence of cerebral infarction. Hebei Medical Journal 2010;32:560-1.
- Wang X. Comparison between Xuesaitong soft capsule and Xinnaotong Capsule in treating cerebral infarction. Shandong Medical Journal 2008;48:125.
- Jia H. Effect of Xuesaitong Soft capsule on carotid atherosclerotic soft plaque and cardiovascular events in senile patients with cerebrovascular disease. Shaanxi Journal of Traditional Chinese Medicine 2017;38:173-5.
- Wang M, Weng Q, Zha Q, et al. Evaluation of clinical efficacy of Xuesaitong Soft capsule in patients with acute lacunar infarction complicated with cerebral microhemorrhage. Chinese Clinical Pharmacology and Therapeutics 2017;22:574-9.
- Mi G, Wang J. Clinical observation on 45 cases of cerebral infarction treated with Xuesaitong soft capsule. Yunnan Journal of Traditional Chinese Medicine and Materia Medica 2009;30:22-3.
- Guo Y, Yang Q, Zhang X. Observation of therapeutic effect of Xuesaitong soft capsule on 40 cases of cerebral infarction. Yunnan Journal of Traditional Chinese Medicine and Materia Medica 2005;26:52.
- Zhao S. Effect of Xuesaitong soft capsule plus acupuncture on lipid metabolism disorder in patients with cerebral infarction. Chinese Journal of Integrated Traditional and Western Medicine 2004;24:1040-1.
- Li P, Liang L. Clinical observation of xuesaitong soft capsule in treating cerebral infarction. Zhejiang Journal of Integrative Medicine 2002;12:146-7.
- Liu L, Jiang C, Wang Y, et al. Effect of Xuesaitong capsule on carotid atherosclerotic soft plaque and hemorheology in senile patients with ischemic cerebrovascular disease. Chinese Journal of Gerontology 2017;37:4524-6.
- Lv J. Application of Xuesaitong soft capsule in rehabilitation period of cerebral infarction. China's Naturopathy 2011;19:47.
- Gao L, Lan H. Xuesaitong soft capsule for treating 45 cases of convalescent ischemic stroke. Chinese Medicine Modern Distance Education of China 2012;10:32-3.
- Yang RF. 33 cases of cerebral infarction treated with Xuesaitong soft capsule. Henan Traditional Chinese Medicine 2006;26:71.
- Zhong S, Zhang H, Huang Y, et al. Treatment of acute cerebral infarction with Xuesaitong soft capsule and its effect on hemorheology. Chinese Remedies & Clinics 2008;8:68-9.
- Zhou D, Cen K, Liu W, et al. Xuesaitong exerts long-term neuroprotection for stroke recovery by inhibiting the ROCKII pathway, in vitro and in vivo. J Ethnopharmacol 2021;272:113943. [Crossref] [PubMed]
- Bai Y, Zhang S, Ren J. Multi-center study of Panax notoginseng total saponin injection in the treatment of cerebral infarction. Chinese Journal of New Drugs and Clinical Remedies 2001;20:257-9.
- Chang D, He Q. Effect of Xuesaitong soft capsule on neurological function and quality of life of patients with cerebral infarction. The channel of pharmaceutical 2017;29:134-6.
- Ai W, Chen Y, Yang Q. Clinical study of Xuesaitong in treatment of severe craniocerebral injury. Journal of Practical Training of Medicine 2003;31:86-8.
- Wang S, Shi J, Sun Y, et al. Reliability and validity of simplified Barthel index in stroke convalescence. Chinese Journal of Rehabilitation 2020;35:179-82.
- Wei X. The traditional Chinese medicine treatment of cerebral infarction. World Health Digest 2011. doi:
10.3969/j.issn.1672-5085.2011.06.410 .10.3969/j.issn.1672-5085.2011.06.410 - Chen H, Chen K. The study on the changes of hemorrheology in patients with acute cerebral bleeding and acute cerebral infarction and their clinical significance. Journal of Chinese Microcirculation 1996;214-5.
- Zhu T, Meng XB, Dong DX, et al. Xuesaitong injection (lyophilized) combined with aspirin and clopidogrel protect against focal cerebral ischemic/reperfusion injury in rats by suppressing oxidative stress and inflammation and regulating the NOX2/IL-6/STAT3 pathway. Ann Palliat Med 2021;10:1650-67. [Crossref] [PubMed]
- Gao Y. The reevaluation of Xuesaitong soft capsule after being marketed. Capital Medicine 2003;10:26-30.





