Quality of life in responders after palliative radiation therapy for painful bone metastases using EORTC QLQ-C30 and EORTC QLQ-BM22: results of a Brazilian cohort
Introduction
Bone metastases are an important cause of mortality and quality of life (QoL) impairment in patients with advanced cancer (1). In addition to lung and liver, the skeleton is one of the most affected site by distant metastasis (2,3). Different skeletal-related events are associated to bone metastasis and known to effectively reduce life expectancy and significantly compromise QoL (4). Pain, pathological fractures, hypercalcemia, bone marrow suppression and neuronal compression are some examples of skeletal-related events, commonly seen in clinical practice.
In the last decade, the European Organization for Research and Treatment of Cancer has developed tools to accurately assess QoL in different oncologic settings. For bone metastasis, the QLQ-BM22 questionnaire was developed and addresses QoL impairment associated with this condition (5). Currently, this instrument has been validated internationally and translated into several different languages, including Portuguese (6). Although the Portuguese version of this questionnaire is available and has shown to be reliable and culturally appropriate, very few studies have used this tool in the Brazilian cancer population.
This study investigated changes in QoL of Brazilian patients with painful bone metastasis who were treated with palliative radiotherapy and systemic therapies.
Methods
Patients from a public and academic hospital in Brazil presenting with painful bone metastasis were prospectively enrolled in this study from September 2014 to October 2015. Accrual started after protocol approval by the research ethics committee. All patients enrolled in this study gave their informed consent. Palliative treatment, either using radiotherapy or chemotherapy, was prescribed by clinical judgment of the attending oncologist.
Inclusion criteria
In this study, patients with bone metastases from known solid tumors presenting with pain scores equal or greater to five were included. Only patients with a Karnofsky performance status (KPS) ≥70 and expected to receive palliative treatment (either radiotherapy or chemotherapy) for pain control were enrolled. Patients who were unable to speak or read Portuguese, younger than 18 years and with KPS less than 70 were excluded from this study.
Treatment protocol and data collection
Baseline data on demographics, primary cancer, KPS, type of treatment, pain medication use and pain scores were collected. All enrolled patients completed the QoL questionnaires. Then, patients were treated with palliative radiotherapy or systemic therapy. Radiation therapy could be prescribed to one or more painful bone sites. Two months after treatment, data on pain scores were re-collected and the QoL questionnaires were re-applied.
Pain levels were graded by an 11-point scale [0–10], and together with use of analgesic medications, were evaluated at two time points: at baseline (before treatment) and two months after palliative treatment. Doses from opioid medications were converted to oral morphine equivalent dose (OMED) by using the opioid equivalence table.
Response to treatment
Treatment response was quantified by the international pain response category (IPRC) using pain scores and opioid dose at baseline and two months after treatment (7). This consensus statement categorizes pain response to a prescribed treatment as complete response (CR), partial response (PR), indeterminate or pain progression by evaluating the difference in opioid doses and pain scores before and after treatment. CR is defined as a post-treatment pain score of 0 with no increase in analgesic intake whereas PR is defined as pain reduction ≥2 points without analgesic increase or analgesic reduction in ≥25% without increase in pain at the treated site. In this analysis, only patients achieving CR and PR were included.
QoL questionnaires
The EORTC has developed and validated instruments to reliably and accurately assess QoL in patients with cancer. This study utilized the EORTC quality of life questionnaire (QLQ)-C30 and QLQ-BM22 instruments (8,9).
The EORTC QLQ-C30 is a cancer-specific tool that broadly assesses QoL in patients with cancer (8). This questionnaire contains 30 items divided into five functional scales (physical, role, cognitive, emotional and social); three symptom (pain, fatigue, nausea and vomiting) domains; two global health and quality of life items; six single items related to common symptoms presented by oncological patients (dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea) and one item related to financial impact of cancer. In each topic, four to seven Likert-type alternative responses are available. After data collection, these numbers are converted to a score ranging from 0 to 100. The validated Portuguese version of the EORTC QLQ-C30 (10) was used in this study.
The EORTC QLQ-BM22 is a specific module that was developed to specifically assess QoL in patients with bone metastases and is applied in conjunction with QLQ-C30 in this clinical setting (9). This questionnaire contains 22 items across both symptom and functional domains. In the symptom domains, five items compose the painful site scale and three items compose the painful characteristics scale. In the functional domain, there are eight functional interference items and six psychosocial items. Each item represents one response divided in a Likert scale from 1 (not at all) to 4 (very much). These numbers were also converted to scores ranging from 0 to 100 and higher scores represent more symptomatology. A validated Portuguese version of the QLQ-BM22 is also available (6,11).
Statistical analysis
Data was reported by using mean, median and quartiles. Non-parametric statistic tests were used in this analysis. The Wilcoxon test was employed for comparison between the pre and post QLQ-C30 and QLQ-BM22 paired values. Results were considered to be statistically significant if P<0.05. The package R version 3.3.0 was used in the statistical analysis.
Results
This study remained open for 13 months and 35 patients were enrolled during this time. All included patients had biopsied known primary cancers and radiological evidence of bone metastases. Table 1 details the demographic and clinical characteristics of the patient cohort. Twenty-three patients were female and the mean cohort age was 56 (range, 22–80) years old. Except for two patients treated with palliative chemotherapy, all others received palliative radiotherapy to the painful site(s).
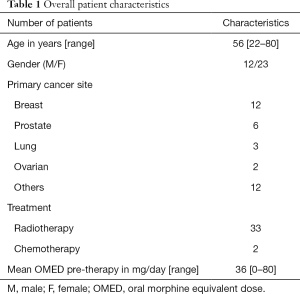
Full table
Two patients died before the two-month reassessment. From the remaining 33 patients alive, 25 achieved a CR or PR pain response in accordance with the IRPC consensus. Table 2 summarizes the characteristics of patients that responded to treatment. All patients with prostate cancer and 92% of patients with breast cancer had pain response to the offered treatment. Eight patients had indeterminate response and none had pain progression.
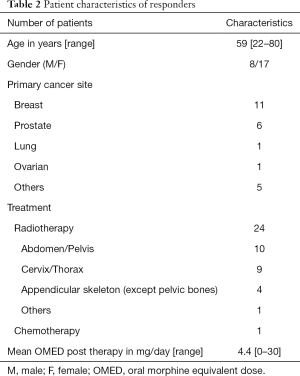
Full table
Treatment responders were evaluated in regards to QoL improvement. From all domains of the QLQ-C30 questionnaire, pain was the only symptom to present a statistically significant difference from baseline to the two months assessment. A reduction of 33 points in the median level of pain was recorded. This reached statistical significance. No other favorable or detrimental impact in QoL was captured by the QLQ-C30 questionnaire (Table 3). The functioning domains (physical, role, emotional, cognitive and social) did not present a statistically significant change post treatment.
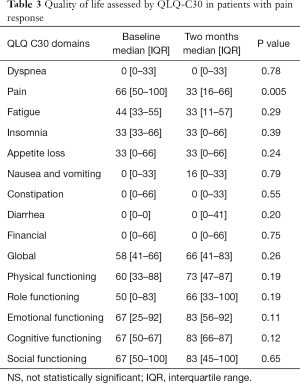
Full table
Overall, the prescribed treatment had a positive impact in responders, as measured by the QLQ-BM22 domains. Except for the psychosocial aspects domain of this questionnaire, a statistical significant improvement in the painful site, pain characteristics and functional interference domains were observed (Table 4).
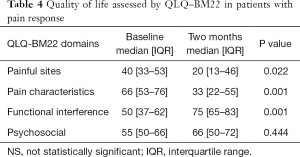
Full table
Discussion
The results of this study are in keeping with other international studies, which show an improvement in the QLQ-BM22 and QLQ-C30 instrument scores after palliative treatment to painful bone metastases (5,6,11,12). The QLQ-BM22 and QLQ-C30 instruments are validated, sensitive instruments for assessing quality of life in patients with bone metastases (8,9). Given that this study included patients who had a CR or PR to treatment, it is expected that pain domain of the QLQ-C30 would be most directly affected. This was the only domain to register a statistically significant difference in the QLQ-C30 instrument.
The difficulty in achieving statistical significance is likely explained by the limited sample size of this study. Other studies have shown similar trends of improved numerical scores across multiple domains but less correlation in the non-conceptually related scales such as financial problems and dyspnea (5,13). It is not readily apparent how treatment of bone metastases would improve quality of life in these domains. Interestingly, the only two domains to register a non-statistical numerical score worsening after treatment were diarrhea and nausea and vomiting. This is in alignment to expected side effects from pelvic/abdomen radiotherapy as 10 patients received radiation to these sites. Also, chemotherapy can also be associated with diarrhea.
The more specific instrument for assessing quality of life issues in patients with bone metastases, the QLQ-BM22, showed a statistically significant improvement in 3 out 4 domains: painful sites, pain characteristics and functional interference. The psychosocial aspects domain also had a numerical improvement but this was not statistically significant. One explanation for this observation is that responders to radiotherapy most readily notice an improvement in their pain and functionality. If these domains are improved, the psychosocial domain improvement may come later and is also affected by factors including disease status and non-cancer related effects. Other studies with larger sample sizes have showed a statistically significant improvement in this domain as well (14).
This study highlights the importance of using a bone metastases-specific instrument for measuring quality of life in this patient population. Despite the limited sample size of this study, the QLQ-BM22 is able to detect statistically significant differences in QoL across most domains, highlighting its sensitivity. The QLQ-C30 is a more general instrument that is also important but tests domains that may not be directly relevant in patients with bone metastases and short follow up intervals, such as financial issues and dyspnea. Previous studies have shown that the focus of the two instruments is different with little cross-correlation between the scores of the individual items (15). Therefore both instruments should be used in a complementary fashion for patients with advanced cancer and bone metastases. This study also shows the applicability of the Portuguese-translated QLQ-C30 and BM-22 instruments in a Brazilian cohort of patients.
Conclusions
The Portuguese version of the QLQ-C30 and the QLQ-BM22 captured changes in pain among patients with bone metastasis treated with radiotherapy or chemotherapy. Pain was the only domain to improve in the QLQ-C30 questionnaire. In the QLQ-BM22, painful site, pain characteristics and functional interference domains were observed to improve after treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Accrual started after protocol approval by the research ethics committee (CAAE: 31670914.1.0000.0065). All patients enrolled in this study gave their informed consent.
References
- Chow E, Bottomley A. Understanding the EORTC QLQ-BM22, the module for patients with bone metastases. Expert Rev Pharmacoecon Outcomes Res 2009;9:461-5. [Crossref] [PubMed]
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 2014;74:210-6. [Crossref] [PubMed]
- Berman AT, Thukral AD, Hwang WT, et al. Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clin Breast Cancer 2013;13:88-94. [Crossref] [PubMed]
- Smith HS. Painful osseous metastases. Pain Physician 2011;14:E373-403. [PubMed]
- Zeng L, Chow E, Bedard G, et al. Quality of life after palliative radiation therapy for patients with painful bone metastases: results of an international study validating the EORTC QLQ-BM22. Int J Radiat Oncol Biol Phys 2012;84:e337-42. [Crossref] [PubMed]
- Chow E, Nguyen J, Zhang L, et al. International field testing of the reliability and validity of the EORTC QLQ-BM22 module to assess health-related quality of life in patients with bone metastases. Cancer 2012;118:1457-65. [Crossref] [PubMed]
- Chow E, Hoskin P, Mitera G, et al. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys 2012;82:1730-7. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Chow E, Hird A, Velikova G, et al. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire for patients with bone metastases: the EORTC QLQ-BM22. Eur J Cancer 2009;45:1146-52. [Crossref] [PubMed]
- Lopes Ferreira P, Pinto Barros A. Measuring quality of life in palliative care. Acta Med Port 2008;21:111-24. [PubMed]
- Miki-Rosário N, Garcia Filho RJ, Garcia JG, et al. Translation into Portuguese, cross-cultural adaptation and validation of "The European Organization for Research and Treatment of Cancer-Quality of Life Questionnaire-Bone Metastases-22". Ann Palliat Med 2016;5:190-5. [Crossref] [PubMed]
- Püsküllüoğlu M, Tomaszewski KA, Bottomley A, et al. Validation of the Polish version of the EORTC QLQ-BM22 module for the assessment of health-related quality of life in patients with bone metastases. Qual Life Res 2014;23:527-32. [Crossref] [PubMed]
- Püsküllüoğlu M, Zygulska AL, Tomaszewska IM, et al. Evaluation of health-related quality of life and its main influencing factors in a Polish population of patients with bone metastases. Curr Probl Cancer 2016;40:183-97. [Crossref] [PubMed]
- McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol 2017;3:953-9. [Crossref] [PubMed]
- Zeng L, Chow E, Zhang L, et al. An international prospective study establishing minimal clinically important differences in the EORTC QLQ-BM22 and QLQ-C30 in cancer patients with bone metastases. Support Care Cancer 2012;20:3307-13. [Crossref] [PubMed]


