Palliation of bone metastases—exploring options beyond radiotherapy
Introduction
While bone metastases can be asymptomatic, they can commonly cause significant morbidity due to pain, pathological fracture and spinal cord compression. The goals of palliative treatment are pain relief, preservation of function, and maintenance of skeletal structural integrity.
Radiation therapy is the mainstay of treatment for symptomatic bone metastases. The primary goal of radiation therapy is to palliate painful bone metastasis, achieve local tumor control and improve quality of life. Typically a palliative dose of conventionally delivered radiation is applied in a convenient scheme such as 8 Gy in a single fraction or 20 to 30 Gy in 5 to 10 fractions. The intent is predominantly pain relief which is thought to be achieved via decreasing the tumor size and surrounding inflammation (1). For bone confined noncomplex metastases, the literature suggests high rates of control; however, for bulky “mass” type tumors tumor control is sub-optimal. This has resulted in the development of high total dose and short course radiation using advanced techniques that allows for dose escalation while respecting the spinal cord known as spine stereotactic body radiotherapy (SBRT). The biologically effective dose can range from 4 to 8 times that of conventional palliative radiation with the intent to improve complete response rates for pain and local control. At our centre, the approach has been to deliver 24 Gy in 2 fractions with mature outcomes reported and this fractionation is currently being evaluated to conventional radiation in a randomized trial [SC-24 phase III randomized trial (NCT02512965)].
Percutaneous vertebroplasty (VP) is a procedure aimed at stabilizing the treated level and alleviating pain in patients with pathological vertebral body fractures (2-4). While VP can mechanically stabilize the vertebral body, it does not treat the local tumor (5). Tumor debulking can be achieved through various locoregional techniques such as thermal ablation, cryotherapy, photodynamic therapy (PDT), embolization (bland or chemoembolization), chemical ablation (alcohol) or external beam radiation. Combining these techniques with VP can treat the tumor and stabilize the fracture in a minimally invasive manner in one setting.
The following is a review of emerging interventional technologies in the palliation of bone metastases. These new techniques are meant to address those patients who have failed conventional lines of therapy and continue to be symptomatic.
Methods
A review of the literature was carried out using the PubMed database and the search terms ‘external beam radiation’ ‘VP’ ‘radiofrequency ablation’ ‘RFA’ ‘bipolar radiofrequency ablation’ ‘bone metastases’ and ‘ablation’. The literature search was carried out August 2017.
Radiation therapy
There are various forms of radiation therapy with the mainstay being short course palliative radiation. There have been multiple randomized trials that have shown high partial response rates at approximately 60% and complete response rates ranging from 10% to 20% (6). The most common regimens include 8 Gy in 1 fraction, 20 Gy in 5 fractions and 30 Gy in 10 fractions. Fracture rates are typically 5% following these regimens but can vary depending on baseline factors as defined by the increasingly applied spinal instability in neoplasia score (7). SBRT has been developed to improve upon the low complete response rates associated with conventional radiation and to improve local control for complex metastases. Data suggest high rates of local control at 1 year ranging from 80% to 90%. At present a phase II randomized trial has been reported comparing 24 Gy in 1 SBRT fraction to 30 Gy in 10 SBRT fractions (8). Importantly this trial was not powered a priori and the result was a trend to higher complete response rates at 3 months at 44% vs. 17%, respectively. Randomized trials are in progress with the appropriate power to answer the question in the palliative patient with painful spinal metastases if spine SBRT is superior.
A downside of SBRT in the spine is an increased risk of vertebral compression fractures. Rates range from 10% to 40% and there is a dose-complication relationship with the highest rates of vertebral compression fracture (VCF) following 24 Gy in 1 fraction (9,10). Other factors that influence risk include lytic disease, baseline VCF, and spinal malalignment (10). A review of the pathophysiology suggest early fractures occurring within the first 3 months associated with the intense edema and inflammation and late fractures associated with a smoldering radiation necrosis. As a result of SBRT, there is increasing use of salvage stabilization interventions upon fracturing, and prophylactic stabilization interventions in high risk patients eligible for SBRT. Evidence is still needed to determine which patients are at the highest risk of developing post-SBRT VCF to justify prophylactic interventions which are most commonly cement augmentation procedures. Another limitation of SBRT lies in the treatment of high-grade epidural disease. SBRT is contraindicated at this time for the treatment of malignant spinal cord compression and there has been an association with epidural disease grade and local control. At present, patients with symptomatic spinal cord compression and single level are potential surgical candidates and if not then conventional palliative radiation is still the standard of care. In those operated upon, post-operative radiation is a standard of care. In keeping with intact metastases, SBRT is also increasingly applied in the post-operative patient. The evidence is limited for this indication and high-quality data is in need as it can be a much more technically difficult application. Ultimately a randomized trial will be needed to clarify the role of post-op SBRT. If symptomatic lesions are widespread such as the prostate patient with a super scan, radiopharmaceuticals or hemibody radiation may be used as palliative alternatives. There is increasing use of radiopharmaceuticals especially for prostate cancer with a recent randomized trial evaluating radium 226 in castrate resistant prostate cancer showed a survival advantage (11).
VP
Polymethylmethacrylate (PMMA) is injected into the bone via percutaneously inserted needles under radiological guidance. PMMA is a commonly used biological cement and has a well-documented safety profile as it is used extensively in hip and knee replacements. The cement flows into spaces as a liquid filling the cavities and then solidifying through an exothermic reaction. Once hardened, PMMA cement is extremely stable and acts as a glue between fragments of bone. In some patients the analgesic effects of VP can be seen rapidly within 24–48 hours (12) while in others it is usually obtained within 2–10 days; often once bruising from the needle access subsides. VP does not reduce tumor burden at the site so it is often paired with other percutaneous locoregional techniques or done in conjunction with radiotherapy.
Kyphoplasty is an analogous technique to VP except that is creates a cavity within the bone using a high-pressure balloon. Liquid cement is then injected into the preformed mechanically created cavity in a very controlled manner. Kyphoplasty enhances control when injecting cement. Radiofrequancy ablation (RFA) assisted VP can provide similar enhancement of control (13). Kyphoplasty has a mechanically created cavity whereas RFA has a cavity created by heat and dessication of the target tissue. Cement can be injected into both cavities safely with decreased incidence of potentially dangerous posterior leaks. There is no high quality, objective evidence that kyphoplasty can permanently restore height or correct spinal curvature. This is important information in managing our patient’s expectations.
Both VP and kyphoplasty are tools in alleviating pain and enhancing structural stability of load bearing bone.
Methods of tumor destruction
Ablative techniques allow for both tumor destruction as well as palliation of pain from metastatic disease (14,15). Tumor destruction can be achieved through a variety of methods.
Thermal ablation
With thermoablative procedures, the goal is to induce coagulative necrosis with heat, ideally around 70 °C for bone lesions (16).
RFA
Currently RFA is the most frequently utilized thermoablative procedure for bone tumors. RFA is dependent on the conductive properties of the target tissue. It works by conducting an alternating current through a probe placed within a lesion (17). This results in the deposition of energy within tissue which induces coagulative necrosis (18,19). Ablation is controlled based on a feedback system dependent on tissue impedance which is often high in bone. RFA devices are usually monopolar or bipolar. Many have features that allow for shaft cooling.
Monopolar RFA has a circuit which is between the electrode tip which acts as the cathode and a grounding pad placed externally on the patient which acts as the anode. The ionic current induces frictional heat production which generates an ablation zone. Downsides of a monopolar system include long ablation times and the ‘heat-sink effect’. The ‘heat-sink’ effect relates to tumors adjacent to large vessels (20,21). When tumor is located adjacent to a large vessel the energy flows from the electrode through the highly conductive liquid to the grounding pad thereby bypassing the tumor (22).
Bipolar RFA creates a circuit between two electrodes in close proximity at the tip of the needle. Energy is then dissipated between the two electrodes ablating the intervening tissue (23). Bipolar systems have faster ablation times, require less power, are less susceptible to the heat sink effect and do not need external grounding pads on the patient. Bipolar RFA has also been shown to produce larger ablation zones when compared to monopolar devices (24).
Internally cooled electrodes use an interior lumen along the shaft of the needle that is filled with a circulating liquid.
This helps to remove heat from the tip of the electrode, prevents charring which can create an insulative sleeve around the needle tip and allows for better heat dispersion which often results in a larger ablation zone. This technique can be used in conjunction with monopolar and bipolar devices.
An example of a monopolar internally cooled radiofrequency probe is the cool-tip electrode (Medtronic). Traditional monopolar devices struggle with bone ablation due to the inherently poor conductive properties of bone. Monopolar systems use an electrical circuit between the placed electrode and a grounding pad in order to create a current which results in heat production and thermal ablation. This common setup used by a monopolar system has a major drawback for use in bone. Osseous tissue has semi-insulative electrical and thermal properties which generates high impedance when completing the circuit from the probe to the grounding pad. This will limit the size of the ablation. Another downside with a monopolar system is that bone marrow within trabeculae may act as a heat sink resulting in a smaller ablation zone. These factors limit the efficacy of monopolar devices in bone applications. Monopolar systems are much better suited to soft tissue ablation where impedance is usually much lower.
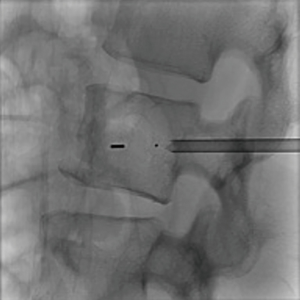
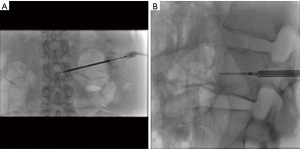
OsteoCool RF Ablation system (Medtronic) is an example of a bipolar system engineered to overcome the limitations of a monopolar device in bone. It is a coaxial bipolar probe with internal cooling. While traditional bipolar systems have required the use of two probes, the OsteoCool probe incorporates the active and grounding electrodes on the tip of a single probe (25). This eliminates the need for placement of a second probe. The benefit of a bipolar system in bone is primarily the ability to bypass the insulative properties of cortical bone. This allows for lower power requirements as the system has less impedance to overcome. The internal cooling component of the device minimizes tissue charring at the probe tip which allows for formation of a larger ablation zone. The 17 gauge probe can be placed through a variety of cannulas with the minimum requirement in size being 13 gauge. Probes come with different active tips; specifically 1, 10 and 20 mm. An independent thermocouple (20 G) can be inserted into sensitive areas to avoid ablation of critical surrounding structures. The generator monitors temperature and moderates power to maintain 70C at the probe tip.
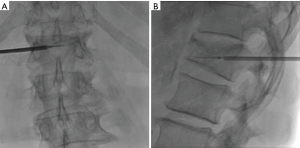
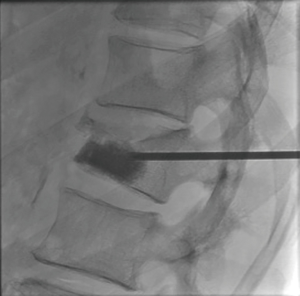
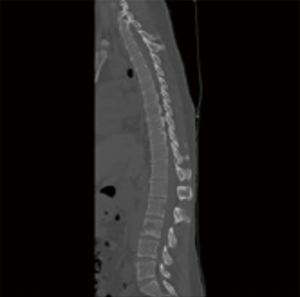
The first study to evaluate the safety of the OsteoCool RF Ablation system was published in 2017. This study determined that RFA assisted VP using a bipolar device was safe (13). RFA allowed for controlled injection of cement into a cavity created by the ablation. There was a significant decrease in venous and posterior cement leaks; presumably due to thrombosis of these vessels during the ablation. Aside from this single center experience trial no other clinical trials with the OsteoCool system have been done at this time. Further clinical evaluation needs to be done in order to understand the long-term impact of bipolar RFA on tumor and bone and whether there are any additional benefits in pairing VP with RFA; such studies are currently ongoing (Figures 1-4).

Cryotherapy
The development of cryoprobes to treat bone lesions is relatively new. Argon gas is delivered through a partially insulated probe which is positioned at the center of a tumor. The gas lowers the probe temperature to −100 °C (26). Cell death is due to loss of cell membrane integrity. An important advantage is that since ice can penetrate bone this can treat osteoblastic metastases. Other advantages include the created ice ball is easily seen by ultrasound (US) or computed tomography (CT). Disadvantages include cryochock which occurs due to the sudden release of tumor cellular contents with the thawing of tissue (2). Other disadvantages include high equipment cost and long operative times.
At this time early evidence does show that cryotherapy is effective at treating primary and metastatic bone tumors. Seventeen tumors in 15 patients were treated with cryoablation using image guidance. This study showed immediate pain relief post procedure with long-term benefits and improvement in quality of life (27).
PDT
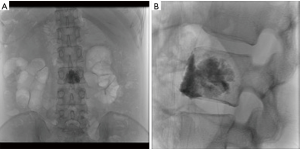
PDT is a relatively new approach to ablation which is not widely available commercially at this point and is mostly utilized in research applications. PDT combines a systemically injected photosensitizing agent with locally applied light at a specific wavelength (typically infrared). The photosensitizing agent accumulates in neoplastic cells over 25 minutes. The agent is then locally activated by light at a low power. Once the agent is activated this generates a cytotoxic singlet oxygen that results in tumor destruction. On a cellular level this occurs due to microvascular injury resulting in tissue hypoxia, infiltration of activated neutrophils and cell apoptosis (28). This efficacy of PDT depends on the light energy which is applied, tissue oxygenation and the optical properties of the tissue. There are many different photosensitizing agents available on the market. At this time, there are no known contraindications to the use of PDT when paired with radiotherapy, RFA or surgery. The use of PDT to treat spinal metastases is currently being investigated; however, early evidence shows that it can safely be used in conjunction with both RFA and VP (29).
PDT can easily be performed in conjunction with VP with the light fiber inserted through the existing bone trocar. At our institution we have safely used RFA in conjunction with PDT. For large lytic lesions which are close to the posterior cortical margin RFA was used in conjunction with PDT to control cement deposition. So far in treatments done usring PDT no neurotoxicity was encountered.
Benefits of PDT include not only ablating vertebral tumors but also the enhancement of vertebral structure (30). Studies have shown periosteal new bone formation within the majority of PDT treated vertebral bodies (31). Other studies have shown that PDT is successful not only destroying vertebral osteolytic tumors but also enhancing vertebral structure particularly when combined with bisphosphonates (32) (Figures 5-9).
Endovascular embolization
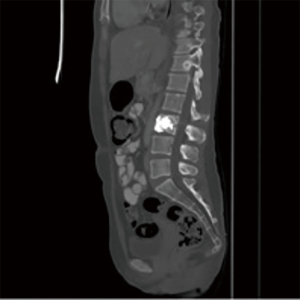
Embolization is the selective occlusion of blood vessels, in this case the blood vessels feeding the tumor. Embolization induces tumor necrosis by occluding these feeding vessels thereby having an analgesic effect. Embolization is typically using calibrated microparticles polyvinyl alcohol (PVA) or trisacryl gelatin microspheres. Chemoembolization combines highly selective arterial embolization with the delivery of intraarterial chemotherapy. Advantages of chemoembolization include that the intraarterial infusion of anti-cancer agents during embolization concentrates the anti-tumor effect 20–40 times and in some cases after destroying osteolytic bone metastases, new healthy bone is deposited (26). The main disadvantage is if the anti-cancer drug comes in contact with nerve roots this can result in neurotoxicity. Arterial embolization of spinal metastases at our institution is used chiefly to devascularize tumors prior to surgical stabilization to minimize intraoperative blood loss.
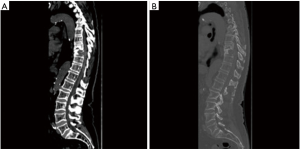
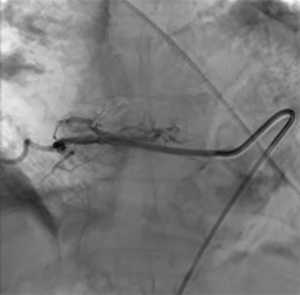
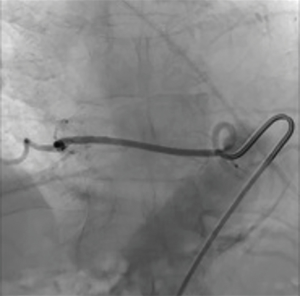
Endovascular embolization is mainly used to devascularize tumors before surgery and as a palliative pain relieving procedure. This is mainly performed for renal cell carcinoma metastases. Pain relief is almost consistently obtained but varies in duration from 3 weeks to 8 months, depending on the aggressiveness of the tumor (33) (Figures 10-12).
Chemical
Percutaneous injection of 95% ethanol in combination with a contrast agent into spinal tumors is a fairly old technique (34). The alcohol induces tumor necrosis (35).
Ethanol created an analgesic effect by destroying the nerve endings adjacent to the tumor thereby providing dramatic and nearly immediate analgesia. In a study of 27 metastatic bone tumors in 25 patients treated with CT guided percutaneous administration of 95% ethanol the outcomes were mixed. Four patients experienced complete relief, 11 had 75% reduction in symptoms while 7 had no relief if pain. This study also found that the analgesic effect does not usually last longer than 3–5 months (34).
Why combine RFA and vertebroplasty
While RFA and verteproblasty can each be used alone to treat spinal metastases there is a potential synergistic effect in combining both (36-38). While RFA alone can reduce tumor burden and control local disease progression, studies have found that RFA treated bone can be less structurally stable. Once tumor is destroyed by any means, radiation, thermal ablation or chemical means; a cavity would have to exist and this would certainly decrease the stability of load bearing bone. The cement adds mechanical stability and potentially prevents future fractures in weakened bone (39,40). Cement injection also appears to be more controlled post RFA especially with posterior leaks and this may be due to tissue dissection and vascular thrombosis that allows cement to be well contained within the cavity created by the ablation (13). An advantage of kyphoplasty over vertebroplasty is the deposition of cement into a mechanically created cavity created by inflation of a high pressure balloon. RFA assisted vertebroplasty allows for controlled cement deposition into a thermally created cavity. There may be additional benefits of combining RFA, radiation and vertebroplasty in increasing time to recurrence but this has to be investigated in future trials.
Surgery
Although the treatment for spinal metastases is largely palliative in certain circumstances spinal surgery can be a part of the treatment algorithm. Rarely curative surgery can be the goal if the spine is the only known site of metastasis. Curative spinal surgery is mainly seen with renal cell carcinoma metastases (41). In other cases, obvious spinal instability, clinically significant neural compression or intractable pain unresponsive to nonoperative measures can require surgery. Generally, surgery is offered to those patients with a life expectancy greater than 3 months and those who would be able to tolerate the procedure (42).
In general, the goals of surgery are to correct and prevent any further deformity by stabilizing the spine and decompressing neural structures.
Conclusions
Locoregional techniques are yet another option in the management of symptomatic bone metastases. Devices for thermal ablation of bone are now widely available on the market. Chemical devices may soon be available. The addition of vertebroplasty to these techniques can improve the mechanical stability of treated bone especially if load bearing. RFA assisted vertebroplasty can also be safely used with more experimental treatments such as PDT. As systemic treatments improve, patients are living longer with bone metastases. In the subset of patients where radiotherapy treatments have been exhausted or are contraindicated, localized techniques paired with cement offer additional options to patients struggling with pain and impaired mobility. The goal in cancer care is not only to live longer but to live with functional status intact and palliating pain is essential to reaching this goal. Locoregional treatments targeting sites of pain and active disease will increasingly take on an important role in palliating patients and maintaining quality of life. All these treatments may also have a synergistic effect when paired with radiotherapy. Further studies are required to establish the optimal sequence of these different interventions in the palliation of bone metastases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yu HH, Hoffee SE. Beyond the conventional role of external-beam radiation therapy for skeletal metastases: new technologies and stereotactic directions. Cancer Control 2012;19:129-36. [Crossref] [PubMed]
- Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: A review. Crit Rev Oncol Hematol 2005;56:365-78. [Crossref] [PubMed]
- Wenger M. Vertebroplasty for metastasis. Med Oncol 2003;20:203-9. [Crossref] [PubMed]
- Callstrom MR, Charboneau J, Goetz M, et al. Painful metastases involving bone: Feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology 2002;224:87. [Crossref] [PubMed]
- Tancioni F. Vertebroplasty for pain relief and spinal stabilization in multiple myeloma. Neurol Sci 2010;31:151-7. [Crossref] [PubMed]
- Sahgal A, Roberge D, Schellenberg D, et al. The Canadian Association of Radiation Oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:629-39. [Crossref] [PubMed]
- Fox S, Spiess M, Hnenny L, et al. Spinal Instability Neoplastic Score (SINS): Reliability Among Spine Fellows and Resident Physicians in Orthopedic Surgery and Neurosurgery. Global Spine J 2017;7:744-8. [Crossref] [PubMed]
- Sprave T, Verma V, Förster R, et al. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother Oncol 2018;128:274-82. [Crossref] [PubMed]
- Faruqi S, Tseng CL, Whyne C, et al. Vertebral Compression Fracture After Spine Stereotactic Body Radiation Therapy: A Review of the Pathophysiology and Risk Factors. Neurosurgery 2018;83:314-22. [Crossref] [PubMed]
- Alghamdi M, Tseng CL, Myrehaug S, et al. Postoperative stereotactic body radiotherapy for spinal metastases. Chin Clin Oncol 2017;6:S18. [Crossref] [PubMed]
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213-23. [Crossref] [PubMed]
- Weill A, Chiras J, Simon JM, et al. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology 1996;199:241-7. [Crossref] [PubMed]
- David E, Kaduri S, Yee A, et al. Initial single center experience: radiofrequency ablation assisted vertebroplasty and osteoplasty using a bipolar device in the palliation of bone metastases. Ann Palliat Med 2017;6:118-24. [Crossref] [PubMed]
- Kurup AN, Callstrom MR. Ablation of skeletal metstases: current status. J Vasc Interv Radiol 2010;21:S242-50. [Crossref] [PubMed]
- Binning MJ, Gottfried ON, Klimo P Jr, et al. Minimally invasive treatments for metastatic tumors of the spine. Neurosurg Clin N Am 2004;15:459-65. [Crossref] [PubMed]
- Ringe KI, Panzica M, von Falck C. Thermoablation of Bone Tumors. Rofo 2016;188:539-50. [Crossref] [PubMed]
- Frangou M, Fourney D. Minimally invasive treatment of spinal tumors. Semin Spine Surg 2009;21:112. [Crossref]
- Ni Y, Mulier S, Miao Y, et al. A review of the general aspects of radiofrequency ablation. Abdom Imaging 2005;30:381-400. [Crossref] [PubMed]
- Lee JM, Choi S, Park H, et al. Radiofrequency thermal ablation in canine femur: Evaluation of coagulation necrosis reproducibility and MRI-histopathologic correlation. AJR Am J Roentgenol 2005;185:661. [Crossref] [PubMed]
- Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency ablation: Does perfusion-mediated cooling limit coagulation necrosis? J Vasc Interv Radiol 1998;9:101-11. [Crossref] [PubMed]
- Khadivar F, Soulen M. Enhancing Ablation: Synergies with Regional and Systemic Therapies. J Vasc Interven Radiol 1998;9:251-6.
- Brace CL, Hinshaw JL, Lubner MG. Thermal ablation for the treatment of abdominal tumors. J Vis Exp 2011;49. [Crossref] [PubMed]
- Lee JM, Choi S, Park H, et al. Radiofrequency thermal ablation in canine femur: Evaluation of coagulation necrosis reproducibility and MRI-histopathologic correlation. AJR Am J Roentgenol 2005;185:661. [Crossref] [PubMed]
- Lee JM, Han JK, Kim SH, et al. Bipolar radiofrequency ablation using wet-cooled electrodes: An in vitro experimental study in bovine liver. AJR Am J Roentgenol 2005;184:391-7. [Crossref] [PubMed]
- Pezeshki PS, Woo J, Akens MK, et al. Evaluation of a bipolar-cooled radiofrequency device for ablation of bone metastases: Preclinical assessment in porcine vertebrae. Spine J 2014;14:361-70. [Crossref] [PubMed]
- Laredo JD, Chiras J, Kemel S, et al. Vertebroplasty and interventional radiology procedures for bone metastases. Joint Bone Spine 2018;85:191-9. [Crossref] [PubMed]
- Masala S, Guglielmi G, Petrella MC, et al. Percutaneous ablative treatment of metastatic bone tumours: visual analogue scale scores in a short-term series. Singapore Med J 2011;52:182-9. [PubMed]
- Burch S, Yee A. Role of Photodynamic therapy for Bone Metastasis. In: Singh G, Rabbani S. Editors. Bone Metastasis. New York: Humana Press, 2005;243-53.
- Pezeshki P. Bone targeted radiofrequency ablation (RFA) electrodes for the treatment of appendicular and vertebral metastases” (Doctoral dissertation). 2011. Available online: https://tspace.library.utoronto.ca/handle/1807/73797
- Fan HT, Wang L, Zhang P, et al. Photodynamic therapy in spinal metastases: a qualitative analysis of published results. Int Surg 2015;100:712-9. [Crossref] [PubMed]
- Won E, Akens MK, Hardisty MR, et al. Effects of photodynamic therapy on the structural integrity of vertebral bone. Spine (Phila Pa 1976) 2010;35:272-7. [Crossref] [PubMed]
- Wise-Milestone L, Akens MK, Lo VC, et al. Local treatment of mixed osteolytic/osteoblastic spinal metastases: is photodynamic therapy effective? Breast Cancer Res Treat 2012;133:899-908. [Crossref] [PubMed]
- Layalle I, Flandroy P, Trotteur G, et al. Arterial embolization of bone metastases: is it worthwhile? J Belge Radiol 1998;81:223-5. [PubMed]
- Gangi A, Kastler B, Klinkert A, et al. Injection of alcohol into bone metastases under CT guidance. J Comput Assist Tomogr 1994;18:932-5. [Crossref] [PubMed]
- Carrafiello G, Laganà D, Pellegrino C, et al. Ablation of painful metastatic bone tumors: a systematic review. Int J Surg 2008;6 Suppl 1:S47-52. [Crossref] [PubMed]
- Hoffmann RT, Jakobs TF, Trumm C, et al. Radiofrequency ablation in combination with osteoplasty in the treatment of painful metastatic bone disease. J Vasc Interv Radiol 2008;19:419-25. [Crossref] [PubMed]
- Munk PL, Rashid F, Heran MK, et al. Combined cementoplasty and radiofrequency ablation in the treatment of painful neoplastic lesions of bone. J Vasc Interv Radiol 2009;20:903-11. [Crossref] [PubMed]
- Clarençon F, Jean B, Pham HP, et al. Value of percutaneous radiofrequency ablation with or without percutaneous vertebroplasty for pain relief and functional recovery in painful bone metastases. Skeletal Radiol 2013;42:25-36. [Crossref] [PubMed]
- Ahn H, Mousavi P, Chin L, et al. The effect of prevertebroplasty tumor ablation using laser-induced thermotherapy on biomechanical stability and cement fill in the metastatic spine. Eur Spine J 2007;16:1171. [Crossref] [PubMed]
- Nakatsuka A, Yamakado K, Maeda M, et al. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol 2004;15:707-12. [Crossref] [PubMed]
- Boriani S, Biagini R, De Iure F, et al. En bloc resections of bone tumors of the thoracolumbar spine. A preliminary report on 29 patients. Spine (Phila Pa 1976) 1996;21:1927-31. [Crossref] [PubMed]
- Hammerberg KW. Surgical treatment of metastatic spine disease. Spine 1992;17:1148-53. [Crossref] [PubMed]