A randomized, double-blind, placebo-controlled study evaluating the efficacy of combination olanzapine, ondansetron and dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving doxorubicin plus cyclophosphamide
Introduction
Breast cancer is the most common cancer among women in Thailand and worldwide (1). Early-stage breast cancer patients usually receive doxorubicin (60 mg/m2) plus cyclophosphamide (600 mg/m2) AC regimen, in adjuvant or neo-adjuvant settings. AC regimen is well established for highly emetogenic chemotherapy (2,3). Chemotherapy-induced nausea and vomiting (CINV) is a major adverse effect of chemotherapy, causing physical and psychological distress (4,5).
Olanzapine is an antipsychotic agent that blocks multiple neurotransmitters, such as dopamine at D1, D2, D3, and D4 receptors; serotonin at 5-hydroxytryptamine type 2a (5-HT2a), 5-HT type 2c (5-HT2c), 5-HT3, and 5-HT type 6 (5-HT6) receptors; catecholamines at alpha1-adrenergic receptors; muscarinic receptors and histamine at H1 receptors in the central nervous system (6-8).
A randomized double-blind, placebo-controlled phase III study showed the significant benefits in CINV prevention from highly emetogenic chemotherapy when adding olanzapine to dexamethasone, neurokinin-1 (NK-1) receptor antagonists and 5-HT3 antagonists (9). The combination of olanzapine, dexamethasone and a single dose of palonosetron also effectively controlled early and delayed CINV (10). The results from a small phase II open-label study demonstrated that olanzapine with ondansetron and dexamethasone reduced the frequency of CINV from highly or moderately emetogenic chemotherapy (11). Thus, olanzapine was recently established a new standard care for CINV prevention since 2016 (9).
Several international guidelines such as those of the National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO) recommended the use of NK-1 receptor antagonists, 5-HT3 receptor antagonists and corticosteroid and/or olanzapine for prevention of CINV after highly emetogenic chemotherapy (2,12,13). Aprepitant was the first NK-1 receptor antagonist that was approved for preventing CINV after highly or moderately emetogenic chemotherapy since 2003. After that, the NK-1 receptor antagonists became the standard of care. Clinical trials of palonosetron which was long-acting 5-HT3 receptor antagonist, demonstrated the superiority of CINV prevention in both the acute and delayed periods when compared with ondansetron 32 mg (14-16). Nowadays, high dose ondansetron 32 mg is not recommended due to cardiac safety (17).
However, most of Thai cancer patients receiving highly emetogenic chemotherapy do not have access to NK-1 receptor antagonists and palonosetron because Thailand is a limited-resource country. Clinical evidence to support the benefits of olanzapine in combination with ondansetron and dexamethasone for CINV prevention is scant because of the time frame of antiemetic drugs’ approval. Therefore, we conducted a randomized double-blind, matching-placebo controlled study to evaluate the efficacy of olanzapine with the real-life practice antiemetic drugs ondansetron and dexamethasone in preventing CINV resulting from AC regimen in early-stage breast cancer patients.
The primary objective was to evaluate the efficacy of olanzapine compared with a matching placebo, in combination with ondansetron and dexamethasone, for control of nausea in early-stage breast cancer patients receiving AC regimen. The secondary objective was to compare the efficacy in terms of a complete response-no emesis and no rescue therapy- between the two study groups.
Methods
Eligibility criteria
Patients in Rajavithi Hospital were included in the study if they were at least 18 years old with histologically confirmed diagnosis of early-stage breast cancer, including invasive ductal and invasive lobular carcinoma. Patients who had previously received chemotherapy were excluded. Early-stage breast cancer patients were eligible for enrollment, if they were scheduled to receive AC regimen (doxorubicin 60 mg/m2 plus cyclophosphamide 600 mg/m2). They needed to have a European Cooperative Oncology Group (ECOG) performance status of 0 or 1 (on a 5-point scale, with 0 indicating no symptoms and higher numbers indicating increasing disability). Additional eligibility criteria were a serum creatinine level of 2.0 mg per deciliter or less, an aspartate or alanine aminotransferase level that was no more than 3 times the upper limit of the normal range, a hemoglobin level of no less than 10 mg per deciliter, a white blood cell count of 3,000 cells per cubic millimeter or more, and an absolute neutrophil count of at least 1,500 cells per cubic millimeter. Pregnant or lactating patients and those who had a history of allergy to planned study drugs were excluded.
Study design and treatment
In this randomized, double-blind, placebo-controlled trial, early-stage breast cancer patients were simply randomized in a 1:1 ratio to receive either olanzapine or a matching placebo with ondansetron and dexamethasone as premedication from the first cycle of AC regimen. In the olanzapine group, patients received olanzapine 10 mg orally on day 1 before chemotherapy and then 10 mg orally once daily on days 2, 3, and 4. In the placebo group, patients received a matching placebo orally in the same schedule. All patients were given ondansetron 8 mg and dexamethasone 20 mg intravenously 30 minutes before chemotherapy administration on day 1 and then oral dexamethasone 10 mg/day on days 2, 3, and 4. Metoclopramide 10 mg was prescribed orally as needed when patients had nausea or vomiting. All patients signed informed consent before enrollment, and this study was reviewed and approved by Rajavithi Hospital ethical review board (Research number 60086). This study received a grant from the Rajavithi Hospital foundation.
Assessment procedures
Patients were requested to complete the daily records of episodes of vomiting or retching (number and time) and their use of rescue therapy (dose and timing) from the first 24 hours of chemotherapy administration through 120 hours for each cycle of AC regimen. Patients were also asked to record daily levels of nausea using a visual-analogue scale ranging from 0 (no nausea at all) to 10 (nausea as bad as it can be) (18). Adverse events were included in the daily records form. An assigned physician contacted each patient by telephone on days 2 through 5 at the first cycle of AC regimen to interview them about potential side effects and remind them to complete the daily record forms. Patients, primary physicians, the assigned physician, and chemotherapy nurses were blinded. All outcomes were analyzed from the self-reported daily record forms of the first cycle of AC regimen. Details of adverse events were collected from all the daily record forms from 4 cycles of AC regimen. Patients remained in their assigned group until 4 cycles of AC regimen were completed or until they withdrew from the study.
Outcomes
The primary endpoint, no nausea rate in the early period, was defined as a response of 0 on a visual-analogue scale for nausea during the early assessment period (0 to 24 hours).
The secondary endpoints were no nausea in the delayed (24- to 120-hour) and overall (0- to 120-hour) periods and a complete response (no emetic episodes and no use of rescue medication). A complete response was determined based on the patients’ daily records during the same overall, early, and delayed assessment periods.
Statistical analysis
A previous publication found that patients who had ondansetron and dexamethasone as premedication for doxorubicin reported a 72% nausea and vomiting rate in the early period (19). In the landmark study, the nausea rate in the early period was 26% in patients receiving olanzapine for prevention of CINV after highly emetogenic chemotherapy (9). We therefore estimated a 46% absolute benefit of nausea prevention from olanzapine. The estimated sample size was 34 patients (17 patients per group) to achieve 90% power to detect this effect size at the 5% significance level (alpha error 0.05), using a two-sided Chi-square test for a fixed sample size. The sample size was increased to 40 patients to compensate for missing data. Patients’ demographic details, nausea rate, complete response rate, nausea scores and adverse events were reported in descriptive analysis. Chi-square test was used to compare the proportion of no nausea and complete response, which were the primary and secondary endpoints respectively, in each assessment period between the two study arms. SPSS version 20.0.0 software was used for all statistical analysis.
Results
Patient characteristics
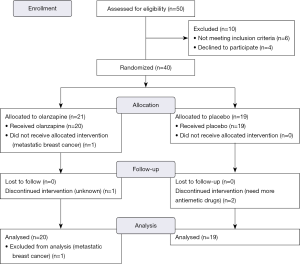
The distribution and randomization of patients was displayed in Figure 1. Between May 2016 and February 2017, we enrolled a total of 40 female patients, 21 and of 19 whom were allocated to the olanzapine group and the placebo group respectively. One patient in the olanzapine group was excluded later as she was found to have asymptomatic liver metastases at presentation. This subject received AC regimen and the study drug for neo-adjuvant treatment before finishing the completed staging.
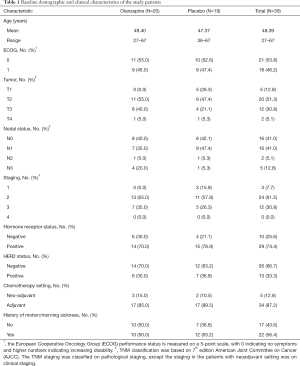
The demographic and clinical characteristics of the 39 patients were presented in Table 1. There were no significant differences between the two groups in terms of age, ECOG performance status, staging, hormonal receptor status, HER2 status, chemotherapy setting, or a history of motion/morning sickness, and no patient had a psychiatric history. The mean ages were 49.40 and 47.37 years old in the olanzapine group and in the placebo group respectively. The majority of patients were diagnosed with stage II breast cancer. AC regimen was mostly prescribed as adjuvant chemotherapy.
Full table
Efficacy
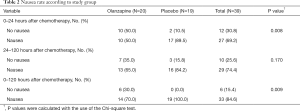
The primary endpoint, the numbers of patients without nausea was showed in Table 2. There were significantly greater proportions of patients without nausea in the olanzapine group than in the placebo group in both the early period (0–24 hours after chemotherapy) and the overall period (0–120 hours after chemotherapy). Patients reporting no nausea in the early period accounted for 50.0% and 10.5% in the olanzapine group and in the placebo group respectively (P=0.008). In the overall period, 30.0% and 0% of patients reported no nausea in the olanzapine and in placebo groups respectively (P=0.009). In the delayed period (24–120 hours after chemotherapy), the proportions of patients without nausea tended to be greater in the olanzapine group than in the placebo group at 35% and 15.8% respectively.
Full table
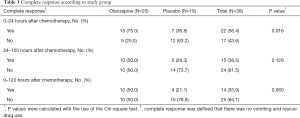
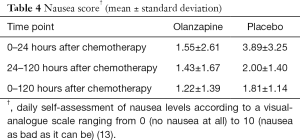
The complete response rate, which was defined as no vomiting or used of rescue medication was presented in Table 3. There was a statistically significant difference in complete response rate between the two treatment groups only in the early period. However, in both the delayed and overall periods, complete response rate tended to be superior in the olanzapine group than in the placebo group. During the early, delayed, and overall periods, the proportions of complete response in the olanzapine group and in the placebo group were 75.0% vs. 36.8% (P=0.016), 50.0% vs. 26.3% (P=0.129), and 50.0% vs. 21.1% (P=0.060) respectively. The mean nausea scores evaluated by a visual-analogue scale were lower in the olanzapine group than in the placebo group in all assessment periods as shown in Table 4.
Full table
Full table
Adverse events
Overall treatment-related adverse events such as insomnia, headache and constipation were not significantly different between the two study groups as demonstrated in Table 5. Somnolence was significantly more common in the olanzapine group than in the placebo group. Increased appetite was not reported in our study. There were two cases of grade 3 febrile neutropenia in the placebo group, but none in the olanzapine group. There was no other serious adverse event. Three patients withdrew from this study after the first cycle of AC regimen: one patient discontinued from the olanzapine group for undisclosed reason while two patients withdrew from the placebo group due to severe nausea that needed antiemetic drugs other than metoclopramide.
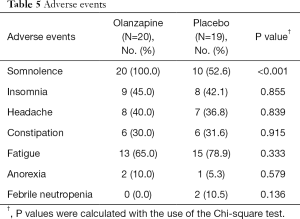
Full table
Discussion
Our randomized, double-blind, placebo-controlled trial confirmed the benefits for nausea prevention in the early period of AC regimen using olanzapine 10 mg adjunctive with Thai real-life practice antiemetic drugs ondansetron and dexamethasone. Olanzapine in this combination was not only able to control chemotherapy-induced nausea at a statistically significant level, but was also clinically meaningful with as much as 40% and 30% superiority in the no-nausea rates in the early period and in the overall period respectively. Patients receiving olanzapine also had 38% fewer episodes of vomiting or need for rescue medication than those in the placebo group in the early period. This study was unable to demonstrate the efficacy of olanzapine in the delayed period CINV prevention. However, there were numerically lower CINV outcomes in terms of no nausea, complete response and nausea score with olanzapine than with the placebo in all assessment periods.
Previous large randomized publications have found that olanzapine with NK-1 receptor antagonist, 5-HT3 receptor antagonist, and steroid prevented CINV incidences in both the acute and delayed periods (9-11). The benefits of using olanzapine with ondansetron in the delayed phase CINV prevention was shown in an open-label study which used a cisplatin-based regimen in advanced non-small lung cancer patients (20). The small sample size in our study most probably accounts for its failure to confirm the benefits of olanzapine in the delayed period. Additionally, our control arm was a less intensive antiemetic regimen than the landmark studies that result in higher CINV in the control arm. For example, in the delayed period, there was only a 75% nausea rate in olanzapine with NK-1 receptor antagonists, 5-HT3 receptor antagonists (mostly palonosetron) and steroid compared to an 84% nausea rate in our study (9). Moreover, we prescribed ondansetron 8 mg which was much lower than ondansetron 32 mg in pivotal trials that showed the superiority of palonosetron for CINV prevention in the delayed phase (14-16). Therefore, long-acting 5-HT3 receptor antagonists could contribute an important role in controlling the delayed period CINV rather than olanzapine alone.
Our inclusion and exclusion criteria aligned with the landmark studies except we did not include high dose cisplatin-based chemotherapy. We decided to choose AC regimen for representing high emetogenic chemotherapy because this regimen was administrated in an out-patient setting which might have reduced unknown confounding factors such as anxiety or anticipatory nausea during hospitalization for high dose cisplatin administration. An out-patient chemotherapy regimen was also suitable for the use of oral antiemetic drugs as control and intervention treatment. Early-stage breast cancer patients alone were enrolled to reduce any potential nausea and vomiting from other causes such as liver metastases. Moreover, breast cancer was the most common cancer in Thai women. We made the decision to focus on the early phase CINV. Because there was a study suggested that adequate CINV prevention in the early phase strongly related to the lower incidence of CINV in the delayed phase (21). In addition, cyclophosphamide in AC regimen induced emesis in a monophasic curve pattern that mostly intense in the first 24 hours which was different from a biphasic curve pattern from cisplatin induced emesis (22).
There was no new adverse event in this study. Somnolence was the major side effect from olanzapine, which is consistent with the findings reported in previous publications. There was a two-fold higher sedative effect in the olanzapine group compared to the placebo group, and all patients receiving olanzapine in our study had episodes of somnolence. Increased appetite was not reported in other clinical trials of olanzapine for CINV prevention (7,8,10). Effect of olanzapine on appetite and weight was reported in the long-term use in psychiatric disorders (23). So, the short-term use of olanzapine for CINV prevention might not increase appetite or body weight.
The limitation of our study was a single-institute study with a small sample size. The external validity might be limited exclusively to early-stage breast cancer patients receiving AC regimen. Olanzapine is not an expensive drug in Thailand due to the availability of generic versions; however, our study did not attempt to address the cost-effectiveness of adding olanzapine to real-life standard antiemetic drugs. Thus, it may not be translated to the public health system and Thai cancer patients might need to incur out-of-pocket expense for olanzapine; nevertheless, it is still more affordable than NK-1 receptor antagonists or palonosetron. For further evaluation, larger sample sizes, more eligible high-emetogenic chemotherapy and multiple-institute studies should be able to confirm the benefits of using olanzapine with ondansetron and dexamethasone. Finally, a study of olanzapine 5 mg compared with olanzapine 10 mg would be helpful regarding sedative effect. The recent phase II study, olanzapine 5 mg had a lower incidence of somnolence than olanzapine 10 mg while the efficacy of olanzapine 5 mg for CINV prevention still remained (24). Phase III clinical trials of olanzapine 5 mg for CINV prevention from high emetogenic chemotherapy are warranted to avoid sedative effects and ensure its efficacy.
In conclusion, our study demonstrated that olanzapine 10 mg combined with ondansetron and dexamethasone was more effective than a placebo in prevention of CINV resulting from AC regimen; doxorubicin (60 mg/m2) plus cyclophosphamide (600 mg/m2), in early-stage breast cancer patients, especially in the first 24 hours after chemotherapy administration.
Acknowledgments
Funding: This study was funded by a grant from the Rajavithi Foundation. This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was reviewed and approved by Rajavithi Hospital ethical review board (research number 60086), and all patients signed informed consent before enrollment.
References
- Imsamran W, Chaiwerawattana A, Wiangnon S, et al. Cancer in Thailand. 8th ed. Bangkok: New Thammada Press, 2015.
- Ettinger DS, Berger MJ, Aston J, et al. NCCN Clinical Practice Guideline in Oncology Antiemesis 2017 Available online: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
- Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2011;29:4189-98. [Crossref] [PubMed]
- Ng TL, Hutton B, Clemons M. Chemotherapy-Induced Nausea and Vomiting: Time for More Emphasis on Nausea? Oncologist 2015;20:576-83. [Crossref] [PubMed]
- Janelsins MC, Tejani MA, Kamen C, et al. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother 2013;14:757-66. [Crossref] [PubMed]
- Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor Binding Profile of the Atypical Antipsychotic Olanzapine. Neuropsychopharmacology 1996;14:87-96. [Crossref] [PubMed]
- Hocking CM, Kichenadasse G. Olanzapine for chemotherapy-induced nausea and vomiting: a systematic review. Support Care Cancer 2014;22:1143-51. [Crossref] [PubMed]
- Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2014;722:180-6. [Crossref] [PubMed]
- Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N Engl J Med 2016;375:134-42. [Crossref] [PubMed]
- Navari RM, Gray SE, Kerr AC. Olanzapine Versus Aprepitant for the Prevention of Chemotherapy-Induced Nausea and Vomiting: A Randomized Phase III Trial. J Support Oncol 2011;9:188-95. [Crossref] [PubMed]
- Mizukami N, Yamauchi M, Koike K, et al. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting in Patients Receiving Highly or Moderately Emetogenic Chemotherapy: A Randomized, Double-Blind, Placebo-Controlled Study. J Pain Symptom Manage 2014;47:542-50. [Crossref] [PubMed]
- Herrstedt J. Antiemetics: an update and the MASCC guidelines applied in clinical practice. Nat Clin Pract Oncol 2008;5:32-43. [Crossref] [PubMed]
- Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119-33. [Crossref] [PubMed]
- Aapro MS. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 2006;17:1441-9. [Crossref] [PubMed]
- Schwartzberg L, Barbour SY, Morrow GR, et al. Pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting (CINV). Support Care Cancer 2014;22:469-77. [Crossref] [PubMed]
- Gralla R. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 2003;14:1570-7. [Crossref] [PubMed]
- Doggrell SA, Hancox JC. Cardiac safety concerns for ondansetron, an antiemetic commonly used for nausea linked to cancer treatment and following anaesthesia. Expert Opin Drug Saf 2013;12:421-31. [Crossref] [PubMed]
- Rapoport B, Schwartzberg L, Chasen M, et al. Efficacy and safety of rolapitant for prevention of chemotherapy-induced nausea and vomiting over multiple cycles of moderately or highly emetogenic chemotherapy. Eur J Cancer 2016;57:23-30. [Crossref] [PubMed]
- Hickok JT, Roscoe JA, Morrow GR, et al. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics. Cancer 2003;97:2880-6. [Crossref] [PubMed]
- Wang X, Wang L, Wang H, et al. Effectiveness of Olanzapine Combined with Ondansetron in Prevention of Chemotherapy-Induced Nausea and Vomiting of Non-small Cell Lung Cancer. Cell Biochem Biophys 2015;72:471-3. [Crossref] [PubMed]
- Schnell FM. Chemotherapy-induced nausea and vomiting: the importance of acute antiemetic control. Oncologist 2003;8:187-98. [Crossref] [PubMed]
- Martin M. The Severity and Pattern of Emesis following Different Cytotoxic Agents. Oncology 1996;53:26-31. [Crossref] [PubMed]
- Tek C, Kucukgoncu S, Guloksuz S, et al. Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early Interv Psychiatry 2016;10:193-202. [Crossref] [PubMed]
- Yanai T, Iwasa S, Hashimoto H, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol 2018;23:382-8. [Crossref] [PubMed]