
Intraoperative use of single dose of nonsteroidal anti-inflammatory drugs was not associated with cancer recurrence and mortality after bladder cancer surgery: a retrospective study
Introduction
Bladder cancer (BCa) is the ninth most common tumor in the world (1). The gold standard for the treatment of high-risk nonmuscle-invasive or muscle-invasive BCa is radical cystectomy (RC). Studies have reported that although surgery has the potential to increase the risk of entry of tumor cells into the blood, most tumor cells are cleared from the circulation (2). Therefore, intraoperative intervention factors potentially have an important effect on postoperative tumor outcomes.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often used for the management of perioperative pain. Preclinical evidence has shown that NSAIDs can regulate the tumor microenvironment by inhibiting inflammatory response (3) and can also participate in the regulation of tumor cell development and progression (4,5). On the basis of these findings, the administration of NSAIDs in the perioperative period might potentially have a significant effect on cancer outcome. In addition, epidemiological studies have found that long-term use of NSAIDs can prevent tumorigenesis (6-9). Recently, intraoperative NSAIDs use was found to be associated with a better prognosis for several tumors (10,11). However, the effect of perioperative NSAIDs use on tumor outcome remains controversial (12-14).
The present study retrospectively evaluated the effect of intraoperative single-dose NSAIDs use on recurrence-free survival (RFS) and overall survival (OS) in patients with BCa who underwent RC. We hypothesized that intraoperative NSAIDs use may be associated with improved tumor prognosis in these patients.
Methods
Study population
The study was approved by the Ethics Committee of the Tenth People’s Hospital of Shanghai (No. SHSY-IEC-4.1/19-120/01). We retrospectively screened 248 patients who underwent RC from January 2009 to October 2018. The inclusion criteria were as follows: patients with transitional cell carcinoma, without secondary malignancies, with complete medical history and follow-up data, and with high-risk nonmuscle-invasive or muscle-invasive BCa. Those patients (n=63) with secondary tumors, non-transitional cell carcinoma, or preoperative metastatic cancer were excluded. Demographical, perioperative, and survival data were collected from electronic medical records, and patients who had received intravenous parecoxib (40 mg) intraoperatively were assigned to the parecoxib group. Patient characteristics included age and sex, American Society of Anesthesiologists (ASA) score, body mass index (BMI), pathologic tumor stage, pathologic lymph node stage, differential grading, number of tumors, size of the largest tumor, prior recurrence status, presence of coexisting carcinoma in situ (CIS), intraoperative blood transfusion, and adjuvant therapy. The neutrophil-lymphocyte ratio (NLR) was calculated as the neutrophil count divided by the lymphocyte count. Preoperative NLR was calculated from routine complete blood counts obtained within 1 week before surgery.
Follow-up plan after surgery was generally recommended once every 3 months for the first 2 years, once every 6 months for the next 2 years, and thereafter once a year. Baseline examinations included history and physical examination, urine cytology, and imaging of the chest abdomen and pelvis. RFS and OS were evaluated as the primary endpoints of this study. RFS was defined as the period from the date of RC to the date of recurrence or death, whichever occurred first. OS was defined as the period from the date of RC to the date of death from any cause. Patients were censored at the last follow-up date if neither recurrence nor death occurred.
Statistical analyses
Continuous variables were analyzed using an unpaired t-test or Mann-Whitney U test. Categorical variables were analyzed using the chi-square or Fisher’s exact tests. The median NLR was 2.6, which was similar to that reported by Viers (15). A cutoff value of 2.6 was used to differentiate patients with high inflammatory status from those with low inflammatory status. The Kaplan-Meier method was used to evaluate RFS and OS. The log-rank test was used to compare the differences in survival between the patient groups. Univariate Cox proportional hazard ratio (HR) models were fitted to evaluate the effects of baseline characteristics on RFS and OS. Multivariate Cox proportional HR models were used to identify the independent predictor by adjusting for any covariates associated with prognosis in previous studies or by using variables with a P value of less than 0.05 in the univariate analyses. A P value of less than 0.05 was considered to be statistically significant. SPSS version 24.0 (SPSS, Chicago, IL, USA) was used for all analyses.
Results
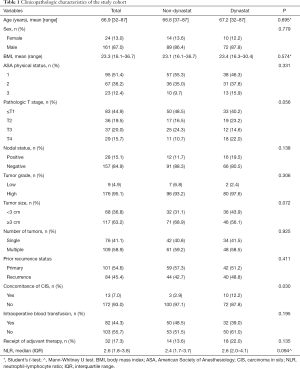
A total of 185 patients were enrolled in the study. The average age of these patients was 66.9 (range, 32–87) years. The majority (87.0%) of patients were males. Of these patients, 12.4% had an ASA score of 3. Almost 50% of the patients had high-risk nonmuscle-invasive BCa. During surgery, 82 patients received a single dose of parecoxib. In the parecoxib group, except for a higher proportion of combined CIS (P=0.030), the other baseline parameters did not differ significantly from those of the non-parecoxib group. There was also no significant difference in the median preoperative NLR between the parecoxib group [2.6; interquartile range (IQR), 2.0–4.1] and the non-parecoxib group (2.4; IQR, 1.7–3.7) (Table 1). The median follow-up time for the overall population was 23.9 months (IQR, 11.9–51.4). Although the parecoxib group had a shorter follow-up time (20.8 vs. 30.9 months, P=0.013), there were no significant differences in cancer-specific mortality, all-cause mortality, and OS between the two groups (Table S1).
Full table

Full table
RFS
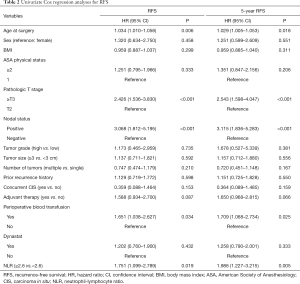
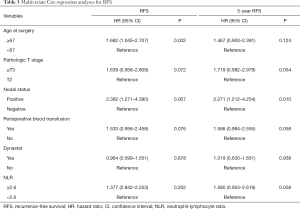
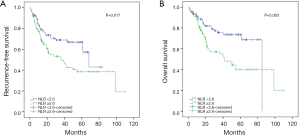
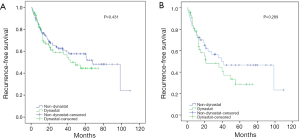
The univariate analysis revealed no association between intraoperative parecoxib use and RFS [HR, 1.202; 95% confidence interval (CI), 0.760–1.900; P=0.432]. Additionally, the analysis showed that age (HR, 1.034; 95% CI, 1.010–1.058; P=0.006), pathologic T stage (HR, 2.426; 95% CI, 1.536–3.830; P<0.001), nodal status (HR, 3.068; 95% CI, 1.812–5.195; P<0.001), perioperative blood transfusion (HR, 1.651; 95% CI, 1.038–2.627; P=0.034), and NLR (HR, 1.751; 95% CI, 1.099–2.789; P=0.019) were associated with RFS. Similarly, the univariate analysis showed that age, pathologic T stage ≥ T3, lymph node positive status, perioperative blood transfusion, and high NLR were still significantly associated with decreased rates of 5-year RFS. However, intraoperative parecoxib use was not associated with 5-year RFS (Table 2). Multivariate analyses further demonstrated that intraoperative parecoxib administration (HR, 0.964; 95% CI, 0.599–1.551; P=0.878) and NLR (HR, 1.377; 95% CI, 0.842–2.253; P=0.202) were not associated with RFS. Instead, the analysis showed that age (HR, 1.682; 95% CI, 1.045–2.707; P=0.032) and nodal status (HR, 2.362; 95% CI, 1.271–4.390; P=0.007) were independent prognostic factors of RFS. Furthermore, pathologic T stage (HR, 1.639; 95% CI, 0.956–2.809; P=0.072) and perioperative blood transfusion (HR, 1.533; 95% CI, 0.956–2.458; P=0.076) showed a trend to decrease RFS. Multivariate analyses also demonstrated that intraoperative parecoxib administration was not associated with 5-year RFS (HR, 1.019; 95% CI, 0.630–1.651; P=0.938). Nodal status (HR, 2.271; 95% CI, 1.212–4.254; P=0.010) was the only independent prognostic factor of RFS. Pathologic T stage (HR, 1.719; 95% CI, 0.992–2.979; P=0.054), perioperative blood transfusion (HR, 1.586; 95% CI, 0.984–2.555; P=0.058), and NLR (HR, 1.580; 95% CI, 0.953–2.619; P=0.058) showed a trend to be associated with 5-year RFS (Table 3). Figure 1 shows the association of NLR with RFS and OS. Figure 2A shows that the use of intraoperative parecoxib had no significant effect on RFS of patients with BCa who underwent RC (P=0.431). The RFS rates at 2 and 5 years were 61.1% and 44.5% in the parecoxib group and 65.0% and 57.6% in the non-parecoxib group, respectively. In the subgroup of patients with high inflammatory status (NLR ≥2.6), no association was observed between intraoperative parecoxib administration and RFS (Figure 2B).
Full table
Full table
OS
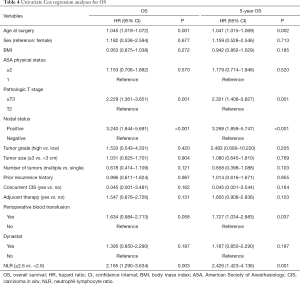
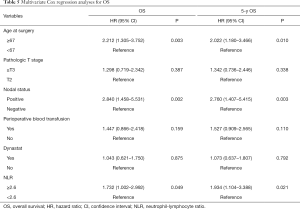
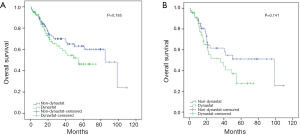
The univariate analysis revealed no association between intraoperative parecoxib use and OS (HR, 1.395; 95% CI, 0.850–2.290; P=0.187). Additionally, the analysis showed that age (HR, 1.045; 95% CI, 1.019–1.072; P=0.001), pathologic T stage (HR, 2.229; 95% CI, 1.361–3.651; P=0.001), nodal status (HR, 3.240; 95% CI, 1.844–5.691; P<0.001), perioperative blood transfusion (HR, 1.634; 95% CI, 0.984–2.713; P=0.058), and NLR (HR, 2.165; 95% CI, 1.290–3.634; P=0.003) were associated with OS. Similarly, the univariate analysis showed that age (HR, 1.041; 95% CI, 1.015–1.069; P=0.002), pathologic T stage ≥ T3 (HR, 2.321; 95% CI, 1.408–3.827; P=0.001), lymph node positive status (HR, 3.268; 95% CI, 1.858–5.747; P<0.001), perioperative blood transfusion (HR, 1.727; 95% CI, 1.034–2.883; P=0.037), and high NLR (HR, 2.426; 95% CI, 1.423–4.136; P=0.001) were significantly associated with decreased rates of 5-year OS. However, intraoperative parecoxib was not associated with 5-year OS (Table 4). Multivariate analyses further demonstrated that intraoperative parecoxib administration (HR, 1.043; 95% CI, 0.621–1.750; P=0.875) was not associated with OS. Instead, the analysis showed that age (HR, 2.212; 95% CI, 1.305–3.752; P=0.003), nodal status (HR, 2.840; 95% CI, 1.458–5.531; P=0.002), and NLR (HR, 1.732; 95% CI, 1.002–2.992; P=0.049) were independent prognostic factors of OS. Multivariate analyses also demonstrated that intraoperative parecoxib administration was not associated with 5-year OS (HR, 1.073; 95% CI, 0.637–1.807; P=0.792). Age (HR, 2.022; 95% CI, 1.180–3.466; P=0.010), nodal status (HR, 2.760; 95% CI, 1.407–5.415; P=0.003), and NLR (HR, 1.934; 95% CI, 1.104–3.388; P=0.021) were independent prognostic factors of 5-year OS (Table 5). As shown in Figure 3A, intraoperative parecoxib use had no significant effect on OS of patients with BCa who underwent RC (P=0.185). The OS rates at 2 and 5 years were 67.4% and 46.3% in the parecoxib group and 72.5% and 63.1% in the non-parecoxib group, respectively. In the subgroup of patients with high inflammatory status (NLR ≥2.6), no association was observed between intraoperative parecoxib administration and OS (Figure 3B).
Full table
Full table
Discussion
Surgical procedures potentially transiently increase the entry of tumor cells into the blood, and intraoperative intervention factors may greatly affect the survival of these tumor cells, leading to postoperative tumor recurrence and metastasis. NSAIDs are a class of perioperatively widely used drugs that are an optional pain reliever in addition to opioid analgesics. Epidemiological investigations have revealed that long-term use of NSAIDs can reduce the incidence of multiple solid tumors (8,9), including BCa (6,7). In addition, NSAIDs have demonstrated antiproliferative and proapoptotic effects in BCa cell lines by inhibiting the enzyme cyclooxygenase-2 (COX-2) (16,17), and a phase II randomized trial showed that perioperative COX-2 and β-adrenergic blockade had favorable effects on metastatic biomarkers in patients with breast cancer, but it did not evaluate the effect on long-term clinical outcomes (18). Intraoperative drugs, including NSAIDs, are chosen only by the anesthesiologist, and the drugs used may critically affect oncological outcomes. From these perspectives, it is very important to investigate the association between intraoperative NSAIDs use and survival after cancer surgery. In the present study, we found that preoperative NLR was a prognostic factor of recurrence and mortality in patients with BCa who underwent surgery. In contrast to these favorable effects of NSAIDs, intraoperative parecoxib was not associated with recurrence or mortality of patients with BCa who underwent RC; similar results were noted for the subgroups with high inflammatory status.
Previous studies have reported conflicting results on the association between perioperative use of NSAIDs and patient survival for several solid tumors. Forget and colleagues found that in patients with kidney, breast, and lung cancers (357, 227, and 255 patients, respectively), NLR and intraoperative use of NSAIDs were strong perioperative prognosis factors (11). Consistent with these results, another retrospective analysis of a single-center cohort also found that intraoperative use of NSAIDs might improve postoperative survival in patients with breast cancer (10). Moreover, a phase-II randomized trial demonstrated that patients receiving NSAIDs before undergoing colon cancer surgery were shown to have a potentially decreased risk of cancer progression and metastasis (18). On the other hand, a retrospective study of 1,637 patients who underwent surgery for non-small cell lung cancer (NSCLC) showed that perioperative NSAIDs use was not associated with increased RFS or OS in these patients as compared to those who did not receive NSAIDs, although preoperative platelet-to-lymphocyte ratio was demonstrated to be associated with decreased rates of RFS and OS (13). Additionally, Choi et al. (12) demonstrated that postoperative NSAIDs use had no significant effect on survival in 1,139 patients with early stage NSCLC, although preoperative NLR was demonstrated to be an independent predictive factor of RFS and OS. Furthermore, in patients with prostate cancer, intraoperative NSAIDs use was found to have no significant effect on improved biochemical RFS (14). In short, the results of the above studies suggest that the effect of perioperative NSAIDs on tumor outcomes may be tumor-specific.
During the last decade, preclinical studies have found that NSAIDs can suppress proliferation and induce apoptotic effects in BCa cells (17). Consistent with these preclinical studies, several pharmaco-epidemiological studies have also documented that long-term use of COX-inhibiting NSAIDs was associated with reduced risk of BCa (6,7). However, the present study failed to demonstrate an association between intraoperative NSAIDs with RFS and OS after BCa surgery. This finding was in contrast with published studies that suggested a positive effect of NSAIDs on oncological outcomes. This may be explained as follows: one explanation could be that a single dose of intraoperative NSAIDs cannot effectively improve the survival of patients with BCa after surgery, which is consistent with epidemiological studies, suggesting that only long-term use of NSAIDs has the effect of reducing the incidence of tumors. One recent pooled analysis from three large prospective cohorts found that regular use (>2 times/week) of nonaspirin NSAIDs, but not nonregular use (≤2 times/week), was associated with a reduction in the risk of BCa (6). In addition, the protective effect of NSAIDs on BCa is potentially dependent on different drug types, including aspirin, nonaspirin nonselective NSAIDs, and selective COX-2 inhibitors (6,7,19). Although COX-2 inhibitors have been reported to suppress bladder tumor growth in mouse and canine models (5,20), the use of celecoxib, a selective COX-2 inhibitor, was found to be associated with an increased risk of BCa (7). This may be partly explained by the findings of a basic study that celecoxib may activate nuclear factor-kappa B (NF-κB) and NF-κB gene transcription (21). It is well known that elevated NF-κB can promote the progression and metastasis of bladder tumors (22).
The present study has several limitations. First, it is a retrospective analysis, with its inherent selection bias. There may also be other uncontrolled and unrecognized biases. Second, the use of intraoperative NSAIDs depends on the preferences of the anesthesiologist, and other intraoperative drugs that may affect tumor prognosis, including sufentanil, clonidine, and ketamine, are potential confounders. Third, the time span of the overall population was large, and many patients still have not reached the end point of follow-up, which affects the reliability of our analysis. Therefore, the results must be interpreted with caution.
Conclusions
The present study found that intraoperative parecoxib use was not associated with improved outcomes after BCa surgery. Elevated preoperative NLR was demonstrated to be associated with RFS and OS in patients with BCa.
Acknowledgments
Funding: This study was funded by the Shanghai Science Committee Foundation (grant number 19411967700) and Natural Science Foundation of China (grant number 81472389, 81672549).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of Shanghai Tenth People’s Hospital (No. SHSY-IEC-4.1/19-120/01) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [Crossref] [PubMed]
- Eschwège P, Moutereau S, Droupy S, et al. Prognostic value of prostate circulating cells detection in prostate cancer patients: a prospective study. Br J Cancer 2009;100:608-10. [Crossref] [PubMed]
- Takada Y, Bhardwaj A, Potdar P, et al. Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-kappaB activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene 2004;23:9247-58. [Crossref] [PubMed]
- Harris RE, Beebe-Donk J, Alshafie GA. Similar reductions in the risk of human colon cancer by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer 2008;8:237. [Crossref] [PubMed]
- Pruthi RS, Derksen E, Gaston K. Cyclooxygenase-2 as a potential target in the prevention and treatment of genitourinary tumors: a review. J Urol 2003;169:2352-9. [Crossref] [PubMed]
- Daugherty SE, Pfeiffer RM, Sigurdson AJ, et al. Nonsteroidal antiinflammatory drugs and bladder cancer: a pooled analysis. Am J Epidemiol 2011;173:721-30. [Crossref] [PubMed]
- Baris D, Karagas MR, Koutros S, et al. Nonsteroidal anti-inflammatory drugs and other analgesic use and bladder cancer in northern New England. Int J Cancer 2013;132:162-73. [Crossref] [PubMed]
- Chan AT, Giovannucci EL, Meyerhardt JA, et al. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 2005;294:914-23. [Crossref] [PubMed]
- García Rodríguez LA, González-Pérez A. Risk of breast cancer among users of aspirin and other anti-inflammatory drugs. Br J Cancer 2004;91:525-9. [Crossref] [PubMed]
- Forget P, Bentin C, Machiels JP, et al. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth 2014;113 Suppl 1:i82-7. [Crossref] [PubMed]
- Forget P, Machiels JP, Coulie PG, et al. Neutrophil: lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol 2013;20 Suppl 3:S650-60. [Crossref] [PubMed]
- Choi JE, Villarreal J, Lasala J, et al. Perioperative neutrophil: lymphocyte ratio and postoperative NSAID use as predictors of survival after lung cancer surgery: a retrospective study. Cancer Med 2015;4:825-33. [Crossref] [PubMed]
- Lee BM, Rodriguez A, Mena G, et al. Platelet-to-lymphocyte ratio and use of NSAIDs during the perioperative period as prognostic indicators in patients with NSCLC undergoing surgery. Cancer Control 2016;23:284-94. [Crossref] [PubMed]
- Forget P, Tombal B, Scholtès JL, et al. Do intraoperative analgesics influence oncological outcomes after radical prostatectomy for prostate cancer? Eur J Anaesthesiol 2011;28:830-5. [Crossref] [PubMed]
- Viers BR, Boorjian SA, Frank I, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol 2014;66:1157-64. [Crossref] [PubMed]
- Okamoto A, Shirakawa T, Bito T, et al. Etodolac, a selective cyclooxygenase-2 inhibitor, induces upregulation of E-cadherin and has antitumor effect on human bladder cancer cells in vitro and in vivo. Urology 2008;71:156-60. [Crossref] [PubMed]
- Klein RD, Van Pelt CS, Sabichi AL, et al. Transitional cell hyperplasia and carcinomas in urinary bladders of transgenic mice with keratin 5 promoter-driven cyclooxygenase-2 overexpression. Cancer Res 2005;65:1808-13. [Crossref] [PubMed]
- Shaashua L, Shabat-Simon M, Haldar R, et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res 2017;23:4651-61. [Crossref] [PubMed]
- Shih C, Hotaling JM, Wright JL, et al. Long-term NSAID use and incident urothelial cell carcinoma in the VITamins and Lifestyle (VITAL) study. Urol Oncol 2013;31:1689-95. [Crossref] [PubMed]
- Grubbs CJ, Lubet RA, Koki AT, et al. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res 2000;60:5599-602. [PubMed]
- Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J 2001;15:2057-72. [Crossref] [PubMed]
- Karashima T, Sweeney P, Kamat A, et al. Nuclear factor-kappaB mediates angiogenesis and metastasis of human bladder cancer through the regulation of interleukin-8. Clin Cancer Res 2003;9:2786-97. [PubMed]


