
Emulsified isoflurane protects beta cells against high glucose-induced apoptosis via inhibiting endoplasmic reticulum stress
Introduction
Diabetes mellitus (DM) is a series of diseases characterized mainly by hyperglycemia; the most common forms of DM are type 1 DM (T1DM) and type 2 DM (T2DM). In 2013, the estimated overall prevalence of DM among adults in China was 10.9% and that of prediabetes was 35.7% (1). As a result of obesity, unhealthy diet, sedentary lifestyle, and genetic factors, the prevalence of T2DM in adolescents and young adults has been dramatically increasing (2).
In T2DM patients, during insulin resistance and impending diabetes, the pancreatic beta cell is capable of synthesizing and secreting sufficient insulin for glucostasis (3). Unfortunately, the excessive synthesis of insulin triggers multiple pathological reactions (4-6), which cause progressive loss of beta cell function, insulin deficiency, and overt T2DM if there are no effective interventions. Endoplasmic reticulum stress (ERS) is an adaptive response during stress, and in T2DM patients, it can be triggered by an imbalance between the folding capacity of the endoplasmic reticulum (ER) and irreparably unfolded and misfolded proteins aggregating in the lumen from excessive proinsulin synthesis (7). Recent studies have confirmed that ERS is involved in the development of insulin resistance and progression to T2DM, and that the inhibition of ERS would protect the function of beta cells (7,8).
Emulsified isoflurane (EIso), which is an emulsified preparation of isoflurane, has marked anesthetic potency and a comparable safety index and safety features as propofol for patients and animals (9,10). Furthermore, research has demonstrated that EIso shows extensive protective effects in organs, including the liver, lung, and kidney (11,12). However, whether EIso can protect the pancreas remains poorly understood. Our previous study showed that EIso administered before ischemia could protect the lung against reperfusion injury through inhibiting ERS pathway (published in Chinese). We therefore hypothesized that EIso could contribute to increasing the beta cell mass by inhibiting ERS.
Methods
Cell culture and treatment
Rat islet beta cell line (RIN-m5F) was purchased from the China Center for Type Culture Collection (CCTCC). The cells were cultured in RPMI-1640 (Gibco, USA) containing 10% fetal bovine serum (FBS) (Gibco, USA) at 37 °C in a 5% CO2 atmosphere. RIN-m5F cells were then randomly divided into five doses of glucose and EIso for different cultures. EIso (8%) was obtained from the Laboratory of Anesthesiology and Critical Care Medicine, West China Hospital of Sichuan University (Chengdu, China). On attaining 70–80% confluency, cells were seeded into 6-well plates at a density of 2×106 and grown overnight. We chose glucose with final concentrations of 0.1M (0.1G group) and 0.3M (0.3G group) to investigate the influence of high glucose to RIN-m5F cells. Based on preliminary experiments, we used 57 µM (0.3G + 57E group) and 76 µM (0.3G + 76E group) EIso to study the EIso protection.
Cell viability assay
Indicated cells were cultured in 96-well plates, followed by drug exposure for 24 h. The rate of cell apoptosis was examined by MTT assay. Briefly, after exposure to glucose and EIso, 200 µL of MTT solution (0.5 mg/mL, Sangon Biotech, Shanghai, China) was subsequently added. After 4 h, 100 µL of dimethyl sulfoxide (DMSO) was added and mixed gently for 10 minutes. Then, the absorbance was measured at 570 nm using a microplate reader.
Insulin level assay
Cells were seeded overnight in 6-well plates and treated as indicated. Insulin levels in the culture medium were quantified using an insulin rat enzyme-linked immunosorbent assay (ELISA) kit (Beyotime Biotechnology, Nantong, China) according to the manufacturer’s instructions.
Caspase-3 activity assay
Caspase-3 activity was measured using the caspase-3 Activity Assay kit (Beyotime Biotechnology, Nantong, China) following the manufacturer’s instructions. In brief, the protein samples from cells were obtained and 100 µg protein was added to a reaction buffer containing Ac-DEVD-pNA (2 mM), and incubated at 37 °C for 2 h. The absorbance of yellow pNA (the cleavage product) was measured with microplate reader at 405 nm. The caspase-3 activity was recorded as the ratio to that of the control group.
Western blotting analysis
After treatment, cellular protein was extracted using RIPA lysis buffer (Beyotime Biotechnology, Nantong, China) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) for 30 min on ice. The supernatant containing soluble total protein was collected after centrifuge at 12,000 rpm for 10 min at 4 °C. The bicinchoninic acid (BCA) protein assay kit (Sangon Biotech, Shanghai, China) was used to evaluate the protein concentrations. Approximately 50 µg of protein was separated by electrophoresis in 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and was subsequently transferred onto nitrocellulose membranes. The membranes were blocked with 5% non-fat milk powder in 0.01M phosphate buffered saline (PBS) (pH 7.4) and 0.05% Tween-20 at room temperature for 1 h. Subsequently, they were incubated with primary antibodies against B cell leukemia/lymphoma 2 (Bcl-2) associated X (Bax), Bcl-2, GPR78, CHOP (Absin Bioscience, Shanghai, China, 1:1,000), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz, USA, 1:1,000) overnight at 4 °C. Membranes were washed 5 times for 5 min with PBS Tween-20 (PBST) and then incubated with the appropriate HRP-conjugated secondary antibody at room temperature for 2 h. The protein expression was detected by enhanced chemiluminescence (ECL) detection kit (Absin Bioscience, Shanghai, China) using G:BOX F3 gel documentation system (Syngene, UK). Quantification of the band intensities was performed with ImageJ.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The expression of mRNA was detected by qRT-PCR. Total RNA was extracted from cells after treatment with Trizol reagents (Invitrogen, USA) according to the manufacturer’s protocol. The concentration of total RNA was measured by spectrophotometer (Thermo Scientific, USA). cDNA was synthesized using a PrimeScript RT reagent kit (Takara, Japan). Then, cDNA was used as a template for qPCR with Premix Ex TaqII (Takara, Japan) on Applied Biosystems 7500 RT-PCR System (Applied Biosystems, CA, USA). The mRNA levels were normalized to GAPDH. Relative quantification was achieved by the comparative 2–ΔΔct method. The nucleotide sequences of the PCR primers were as follows (5' to 3'): GRPDH forward-AGTGCCAGCCTCGTCTCATA, GRPDH reverse-TACGGCCAAATCCGTTCACA; activating transcription factor-6 (ATF6) forward-GAGTCGCCTTTTAGTCCGGT, ATF6 reverse-ACTCCCAGTCTTCACCTGGTC; X-box-binding protein 1 (Xbp1) forward-GTCCGCAGCACTCAGACTAC; Xbp1 reverse-ATCTGAAGAGGCAACAGCGT; eukaryotic translation initiation factor-2α (eIF2α) forward-GCAATGGAGAAAATTTGCCTTGA, eIF2α reverse-TCATCTGACCAGGAAGGACAC (Sangon Biotech, Shanghai, China).
Statistical analysis
Data were analyzed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Data were presented as mean ± standard deviation. Different experimental groups were compared by Student’s t-test. Two-tailed values of P<0.05 were considered statistically significant.
Results
EIso promoted cell viability and restored the function of high glucose-induced RIN-m5F
We evaluated the effect of high glucose on the morphology of RIN-m5F cells. It was revealed that cell shrinkage appeared after exposure to 0.1M and 0.3M glucose for 24 h, and the quantity and rate of cellular attachment were reduced. Cell shrinkage and round off were significantly decreased, while the quantity and rate of cellular attachment were significantly increased when 57 or 76 µM EIso was added (Figure 1A).
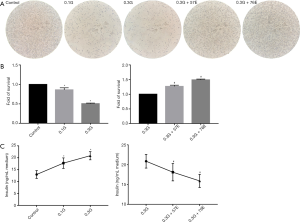
In addition, an MTT assay was performed to investigate cell viability, and demonstrated that high glucose in the culture media induced significant loss of cell viability in a concentration-dependent manner. Treatment with the 0.1M glucose significantly decreased the viability of RIN-m5F cells compared with the control (0.9603±0.052, P<0.05). Cell viability of 0.3G group was also markedly decreased compared to the control group (0.5061±0.0140, P<0.05). Compared to the 0.3G group, 57 µM EIso (1.2717±0.0392, P<0.05) improved the survival of RIN-m5F, and 76 µM EIso (1.4935±0.0262, P<0.05) also improved the survival (Figure 1B), indicating that EIso improved the survival of RIN-m5F cells.
Furthermore, we assessed the impact of EIso on the insulin secretion of RIN-m5F cells, by measuring the insulin concentration in the culture medium after 24 h treatment. The results suggested that the insulin secretion was significantly enhanced after 24 h cultivation with 0.1M (17.76±2.20, P<0.05) and 0.3M (20.88±1.72, P<0.05) glucose compared with the control group (12.98±1.65). In contrast, cells with 57 µM (18.12±2.19, P<0.05) and 76 µM (15.92±1.66, P<0.05) EIso maintained the insulin secretion capacity compared with the 0.3G group (Figure 1C).
Taken together, our result indicated that EIso showed protective capacity in a dose-dependent manner in high glucose-induced cell injury in RIN-m5F.
EIso protected RIN-m5F against glucose-induced apoptosis
The above results showed high glucose diminished the viability of RIN-m5F, and the reduction was reversed by treatment with EIso. We then investigated the mechanism of high glucose damage. First, Ac-DEVD-pNA was used as the catalytic substrate for caspase-3 activity detection. Compared to the control group, caspase-3 activity was increased in the 0.1G group (1.72±0.10, P<0.05), and reached a maximum value (1.97±0.16, P<0.05) in the 0.3G group. After EIso treatment, compared to the 0.3G group, caspase-3 activity decreased in the 0.3G + 57E group (0.91±0.02, P<0.05), and was markedly decreased in the 0.3G + 76E group (0.70±0.15, P<0.05) (Figure 2A).
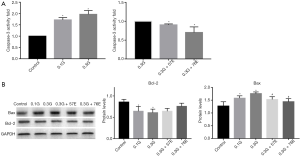
In addition to this, we detected the expression of biomarkers of apoptosis: Bcl-2 and Bax. Results showed that, in the 0.1G group (1.54±0.11, P<0.05), the high glucose environment induced higher Bax expression than in the control group (1.27±0.17), and was significantly increased in the 0.3G group (1.76±0.06, P<0.05) relative to the control group. In contrast, Bax expression was inhibited by treatment with 57 µM (1.53±0.11, P<0.05) and 76 µM (1.44±0.12, P<0.05) EIso for 24 h compared to the 0.3G group. Compared to control group, high glucose environment induced Bcl-2 expression decrease in a concentration-dependent manner (0.86±0.06 vs. 0.64±0.11, 0.86±0.06 vs. 0.61±0.08, P<0.05). However, no difference was detected between the 0.3G and EIso treatment groups (Figure 2B).
EIso alleviates apoptosis of RIN-m5F by inhibiting ERS
To verify the hypothesis that ERS is involved in the protection of EIso, we detected the expression of GRP78 and CHOP. Compared to the control group, the culture media enhanced the expression of CHOP in the 0.1M glucose (0.37±0.06 vs. 0.61±0.07, P<0.05) and the 0.3G group (0.37±0.06 vs. 0.87±0.07, P<0.05). The expression of GRP78 showed no difference between the control group and the 0.1G group (0.60±0.06 vs. 0.70±0.08, P>0.05); however, GRP78 expression was significantly increased in the 0.3G group compared with the control group (0.60±0.06 vs. 0.83±0.05, P<0.05). To our excitement, all the expressions of GRP78 and CHOP were inhibited with EIso treatment for 24 h. Respectively, compared to the 0.3G group, GRP78 expression was significantly decreased in 57 µM (0.83±0.05 vs. 0.66±0.06, P<0.05) and 76 µM (0.83±0.05 vs. 0.45±0.06, P<0.05) EIso; the CHOP expression also decreased in 57 µM (0.87±0.07 vs. 0.46±0.10, P<0.05) and 76 µM (0.87±0.07 vs. 0.31±0.10, P<0.06) EIso (Figure 3A).
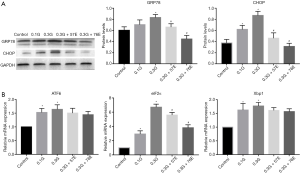
Finally, we detected the expression of the element of the ERS canonical signaling pathway: ATF6, Xbp1, and eIF2α. Results showed that high glucose increased the mRNA expression of ATF6, Xbp1, and eIF2α. Compared with the control group, mRNA expression of ATF6 increased in the 0.1G (1.53±0.15, P<0.05) and in the 0.3G (1.64±0.16, P<0.05) groups, while mRNA expression of Xbp1 increased in the 0.1G (1.62±0.24, P<0.05) and in the 0.3G (1.77±0.13, P<0.05) groups; the mRNA expression of eIF2α increased in the 0.1G (2.94±0.44, P<0.05) and 0.3G (6.71±0.43, P<0.05) groups. After being treated with EIso for 24 h, the expression of eIF2α mRNA was inhibited in a concentration-dependent manner. Compared to the 0.3G group, the expression of eIF2α mRNA was significantly decreased by 57 µM EIso (6.71±0.43 vs. 5.61±0.36, P<0.05) and 76 µM (6.71±0.43 vs. 3.81±0.41, P<0.05) EIso. However, there were no differences in the concentration of 0.3G and EIso treatment when ATF6 and Xbp1 mRNA were detected (Figure 3B).
Our data suggested that the effect of glucose-induced apoptosis in RIN-m5F cells could be restored by EIso at a certain concentration.
Discussion
Glucotoxicity, which refers to the irreversible damage of islet beta cells, plays an important role in the development of DM (13). There is copious research concerning glucose-induced apoptosis in rodent primary islet cells, INS-1 cells, and MIN6 cells (14,15). However, few articles have given an account of rat islet beta cell RIN-m5F. Thus, in this study, we evaluated the damage of glucose on RIN-m5F. Our results showed that 0.1M and 0.3M glucose stimulated the secretion of insulin, altered the morphology, and decreased the viability within 24 h treatment. Kornelius also revealed that the exposure of RIN-m5F cells to high glucose and free fatty acid (FFA) increases apoptosis (16). While the effect of glucotoxicity on RIN-m5F is still fairly well understood, we sought clarify the protective effects of EIso against glucotoxicity.
Isoflurane is an isomeride of enflurane, which is commonly used in inhalation anesthesia, but it can also induce neurogenetic damage and neurocognitive disorder, and even accelerate the process of Alzheimer’s disease (17-19). With a view to identifying the adverse effects of inhalational anesthetic compared to intravenous anesthetic, EIso was manufactured by the West China Hospital of Sichuan University, China, and liquid isoflurane was mixed with intralipid at a final concentration of 8% (v/v). Except for marked anesthetic potency and safety, Hu demonstrated that EIso pretreatment protects the myocardial ischemia and reperfusion injury in rats, and might be mediated by the inhibition of apoptosis and cell damage (20). In this study, we found that EIso protects the morphology and enhances the viability of RIN-m5F while restoring the synthesis of insulin in 0.1M and 0.3M glucose treatment. Additionally, our results showed that EIso inhibits the activity of caspase-3. Caspase-3 is a predominant player in the execution of apoptosis, which can target key structural and regulatory proteins to bring about apoptotic cell death (21). Cao indicated that caspase-3 is critical for the induction of MIN6 cells apoptosis and its activation is further confirmed to be related to NF-κB-mediated Bcl-2 down-regulation (22). Bcl-2 family proteins are the key to the regulation and execution of apoptosis, among which Bcl-2 is a pro-survival protein, and Bax is a pro-apoptotic protein (23). In this study, we found that high glucose-induced Bcl-2 expression decreased and Bax expression increased, but EIso down-regulated the expression of Bax and up-regulated the expression of Bcl-2. It thus seems that EIso protecting RIN-m5F against glucotoxicity may by relevant to apoptosis.
The mechanisms of apoptosis are highly complex, as they involve a cascade of molecular events. Increased protein synthesis, imbalance of ER calcium levels, glucose and energy deprivation that trigger ERS can induce beta cell failure by unfolded protein responses (UPRs) (24). GRP78 and CHOP are the best studied inducers of ERS. GRP78 is an important sensor of misfolded proteins in the ER lumen. When misfolded proteins accumulate, GRP78 is competitively bound by misfolded proteins with EIF2AK3, which results in protein kinase RNA (PKR)-like ER kinase (PERK) activation and phosphorylation of eIF2α (25). An increased availability of GRP78 may reestablish the homeostasis of ER by attenuating UPR (26). UPR protects cells from stress and contributes to the re-establishment of ER; however, prolonged UPR promotes CHOP expression. CHOP induces the expression of growth arrest and DNA damage 34 (GADD34), activates death receptor 5 (DR5), and decreases Bcl-2 anti-apoptotic protein (27). In this study, we demonstrated that EIso increased the expression of GEP78 both in the concentration of 57 and 76 µM with 24 h treatment. In addition, EIso decreased CHOP expression in 57 and 76 µM. ERS triggers downstream response by three canonical signaling sensors: PERK, inositol-requiring enzyme 1α (IRE1α), and ATF6 (28). Next, we detected the mRNA expression of ATF6, Xbp1, and eIF2α. Xbp1 is a molecule that is mediated by the activation of IRE1α. Our results showed that high glucose increased the protein expression of ATF6, Xbp1 and eIF2α, and eIF2α mRNA expression decreased after being treated with 57 and 76 µM EIso. These results provide evidence indicating that EIso may protect RIN-m5F by partly inhibiting ERS.
Conclusions
The current study demonstrated the ERS-mediated apoptosis was induced by high glucose in rat islet beta cell RIN-m5F. EIso promoted RIN-m5F function and anti-apoptosis by inhibiting ERS. The application of EIso during operations on DM patients may protect the function of the pancreas and impede the stress hyperglycemia.
Acknowledgments
Funding: This work was supported by the Youth Fund of the Wuxi Commission of Health (Q201715, Q201819). All the authors thank this fund and the Wuxi People’s Hospital of Nanjing Medical University, Wuxi Children’s Hospital for providing experimental equipment support for the study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017;317:2515-23. [Crossref] [PubMed]
- Lascar N, Brown J, Pattison H, et al. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol 2018;6:69-80. [Crossref] [PubMed]
- DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers 2015;1:15019. [Crossref] [PubMed]
- Pearson G, Soleimanpour SA. A ubiquitin-dependent mitophagy complex maintains mitochondrial function and insulin secretion in beta cells. Autophagy 2018;14:1160-1. [Crossref] [PubMed]
- Abedini A, Cao P, Plesner A, et al. RAGE binds preamyloid IAPP intermediates and mediates pancreatic β cell proteotoxicity. J Clin Invest 2018;128:682-98. [Crossref] [PubMed]
- Biden TJ, Boslem E, Chu KY, Sue N. Lipotoxic endoplasmic reticulum stress, β cell failure, and type 2 diabetes mellitus. Trends Endocrinol Metab 2014;25:389-98. [Crossref] [PubMed]
- Cakir I, Nillni EA. Endoplasmic reticulum stress, the hypothalamus, and energy balance. Trends Endocrinol Metab 2019;30:163-76. [Crossref] [PubMed]
- Salvadó L, Palomer X, Barroso E, et al. Targeting endoplasmic reticulum stress in insulin resistance. Trends Endocrinol Metab 2015;26:438-48. [Crossref] [PubMed]
- Huang H, Li R, Liu J, et al. A phase I, dose-escalation trial evaluating the safety and efficacy of emulsified isoflurane in healthy human volunteers. Anesthesiology 2014;120:614-25. [Crossref] [PubMed]
- Zhou JX, Luo NF, Liang XM, et al. The efficacy and safety of intravenous emulsified isoflurane in rats. Anesth Analg 2006;102:129-34. [Crossref] [PubMed]
- Qin Z, Lv E, Zhan L, et al. Intravenous pretreatment with emulsified isoflurane preconditioning protects kidneys against ischemia/reperfusion injury in rats. BMC Anesthesiol 2014;14:28. [Crossref] [PubMed]
- Zhang L, Luo N, Liu J, et al. Emulsified isoflurane preconditioning protects against liver and lung injury in rat model of hemorrhagic shock. J Surg Res 2011;171:783-90. [Crossref] [PubMed]
- Del Prato S. Role of glucotoxicity and lipotoxicity in the pathophysiology of type 2 diabetes mellitus and emerging treatment strategies. Diabet Med 2009;26:1185-92. [Crossref] [PubMed]
- Kooptiwut S, Hanchang W, Semprasert N, et al. Testosterone reduces AGTR1 expression to prevent β-cell and islet apoptosis from glucotoxicity. J Endocrinol 2015;224:215-24. [Crossref] [PubMed]
- Chen F, Sha M, Wang Y, et al. Transcription factor Ets-1 links glucotoxicity to pancreatic beta cell dysfunction through inhibiting PDX-1 expression in rodent models. Diabetologia 2016;59:316-24. [Crossref] [PubMed]
- Kornelius E, Li HH, Peng CH, et al. Liraglutide protects against glucolipotoxicity-induced RIN-m5F β-cell apoptosis through restoration of PDX1 expression. J Cell Mol Med 2019;23:619-29. [Crossref] [PubMed]
- Wang N, Lu Y, Wang K, et al. Simvastatin attenuates neurogenetic damage and improves neurocongnitive deficits induced by isoflurane in neonatal rats. Cell Physiol Biochem 2018;46:618-32. [Crossref] [PubMed]
- Zuo CL, Wang CM, Liu J, et al. Isoflurane anesthesia in aged mice and effects of A1 adenosine receptors on cognitive impairment. CNS Neurosci Ther 2018;24:212-21. [Crossref] [PubMed]
- Perucho J, Rubio I, Casarejos MJ, et al. Anesthesia with isoflurane increases amyloid pathology in mice models of Alzheimer’s disease. J Alzheimers Dis 2010;19:1245-57. [Crossref] [PubMed]
- Hu ZY, Luo NF, Liu J. The protective effects of emulsified isoflurane on myocardial ischemia and reperfusion injury in rats. Can J Anaesth 2009;56:115-25. [Crossref] [PubMed]
- Wu H, Che X, Zheng Q, et al. Caspases: a molecular switch node in the crosstalk between autophagy and apoptosis. Int J Biol Sci 2014;10:1072-83. [Crossref] [PubMed]
- Cao ZH, Yin WD, Zheng QY, et al. Caspase-3 is involved in IFN-γ- and TNF-α-mediated MIN6 cells apoptosis via NF-κB/Bcl-2 pathway. Cell Biochem Biophys 2013;67:1239-48. [Crossref] [PubMed]
- Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 2019;20:175-93. [Crossref] [PubMed]
- Pagliassotti MJ, Kim PY, Estrada AL, et al. Endoplasmic reticulum stress in obesity and obesity-related disorders: an expanded view. Metabolism 2016;65:1238-46. [Crossref] [PubMed]
- Kepp O, Semeraro M, Bravo-San Pedro JM, et al. eIF2α phosphorylation as a biomarker of immunogenic cell death. Semin Cancer Biol 2015;33:86-92. [Crossref] [PubMed]
- Liu L, Chowdhury S, Fang X, et al. Attenuation of unfolded protein response and apoptosis by mReg2 induced GRP78 in mouse insulinoma cells. FEBS Lett 2014;588:2016-24. [Crossref] [PubMed]
- Fernández A, Ordóñez R, Reiter RJ, et al. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res 2015;59:292-307. [Crossref] [PubMed]
- Villalobos-Labra R, Subiabre M, Toledo F, et al. Endoplasmic reticulum stress and development of insulin resistance in adipose, skeletal, liver, and foetoplacental tissue in diabesity. Mol Aspects Med 2019;66:49-61. [Crossref] [PubMed]


