
Plasma parameters and risk factors of patients with post-stroke cognitive impairment
IntroductionOther Section
With a population that has an increased amount of aged individuals, there have been great improvements to the survival rate of individuals with cardiovascular disease and cerebrovascular disorders, as well as the prolongation of human life; however, the number of people suffering from dementia has increased dramatically. Due to a stroke being a major cause of dementia, the prevalence of dementia will rise in the future due to the increase of stroke incidences and a decrease in mortality among elderly people. Post-stroke dementia (PSD) leads to physical disability, and it decreases the patients’ quality of life. Therefore, PSD has become one of the most serious public health problems worldwide.
Given the substantial health and economic burden of PSD, the early or timely identification of dementia has become a priority in many countries. More recently (1), cognitive decline has been identified even prior to the stage of dementia, while mild cognitive impairment related to vascular lesions has been identified as the preferred target of therapeutic strategies in order to slow or stop the decline, thus avoiding progression towards dementia and related loss of autonomy in daily living.
Post-stroke cognitive impairment (PSCI) was a major complication of a stroke, which has numerous risk factors associated with it. A scale designed to classify the risk of cognitive impairment 6 months after a mild stroke will be useful for clinicians as a guideline. Therefore, it will be useful to develop a risk score based on clinical and plasma biochemical parameters to identify mild ischemic stroke patients at high risk for PSCI. PSCI encompasses the whole spectrum of cognitive disorder after stroke, including post-stroke mild cognitive impairment and PSD. As the prevalence of stroke is high, it is necessary to evaluate the risk of PSCI among stroke patients so that preventive measures and rehabilitation therapy can be used to target those most at risk. Prevention and treatment of PSCI are critical priorities for both clinical care and scientific research. The growing health, social, and economic burden of PSCI is driving the demand for clinical studies that evaluate the benefits and risks of both pharmacological and non-pharmacological interventions (2).
Although many studies explored the incidence and correlated factors of cognitive impairment after stroke (3-6), the mechanism for PSCI is unclear, and the relationship with plasma biochemical indicators of PSCI remains uncertain.
In this study, we investigated the plasma biochemical parameters of PSCI and its potential risk factors.
MethodsOther Section
Patients
From 1 January 2014 to 12 October 2016, we recruited 487 inpatients and outpatients with a stroke in the First People’s Hospital of Bengbu Medical University. Among these patients, 241 of them were male. The mean age of these patients was 71.51±10.15 years (range: 55–80 years).
Inclusion criteria of patients with PSCI
- Available medical and neurological history.
- Presence of acute or subacute nervous system symptoms and signs of PSCI.
- Dementia diagnosed using the criteria of the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) and the International Classification of Diseases-10 (ICD-10).
- History of coronary heart disease, arterial hypertension, hyperlipidemia, or diabetes.
- Fluctuating course or progressive development.
- Hachinski ischemia scale score >7.
- Computed tomography (CT) and magnetic resonance imaging (MRI) revealing multiple cortical or subcortical ischemic brain lesions.
All the patients were followed up with for 3 years after a stroke. We used the Mini Mental State Examination (MMSE) score, Montreal Cognitive Assessment Scale (MoCA) score, clinical dementia rating (CDR), activities of daily living of these patients. Patients with mild dementia can often still make judgments about society and social interactions. They could communicate with people and make decisions for themselves, such as whether to participate in a research study. For patients with moderate or severe dementia, as well as those with severely damaged social, social communication skills, and judgment ability, the patient’s legal representative decided whether to provide consent for the patient’s participation in the study.
After patients were selected, we explained to them (or their representatives) the purpose and significance of our study and the possible benefits and risks. All eligible patients (or their representatives) were informed that participation in the study was voluntary and that they could withdraw from the study at any time. Thus, all included participants, or their representative, provided written informed consent.
All included participants underwent brain CT in the acute phase. Additionally, 397 patients (81.7%) underwent MRI 3 months later. White matter lesion (WML) was defined as none to mild, moderate, or severe, in accordance with the Leukoaraiosis and Disability in the Elderly Study (7).
All patients underwent a detailed systemic and neurological examination, routine laboratory examinations (e.g., blood biochemistry test including electrolyte and lipid panels, blood sugar, complete blood count), electrocardiography (EKG), chest radiography, color ultrasound diagnostic imaging of atherosclerotic plaques in the carotid and vertebral arteries, and echocardiography to identify cardiac sources of emboli and other cardiac disorders.
Clinical evaluation
Medical and neurological histories were recorded, as described previously. In the basic cohort of 487 patients, there were 339 (69.6%) patients with first-ever stroke and 151 patients (31%) with recurrent stroke. Stroke severity was assessed using the modified Rankin score at 3 months (8). Patients’ educational level was divided into two categories: low (0–6 years of formal education) and high (>6 years of formal education). Smoking habits were dichotomized as follows: nonsmoking and smoking (current or former). Alcohol use was categorized on admission as non-alcohol user and alcohol user (past or current alcohol use).
All available hospital records were reviewed to determine whether patients had a history of hyperlipidemia, atrial fibrillation, arterial hypertension, or diabetes. History of hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg. Diabetes was considered if the patient had a previously documented diagnosis with fasting blood sugar ≥110 mg/dL or if the patient was currently under antidiabetic or insulin treatment.
Clinical cognitive assessment was performed using the MMSE and MoCA to measure the level of cognitive dysfunction. The neurological assessment included attention, orientation, language tests, and structuring subtests.
The severity of stroke was determined according to the National Institute of Health Stroke Scale. Furthermore, social functioning after stroke was assessed using the Index of Activities of Daily Living (ADL).
The study was approved by the Ethics Committee of the First People’s Hospital of Bengbu Medical College (approved date: 2013-11-10).
Data analysis and statistics
All data were analyzed using IBM SPSS 21.0 statistical software (IBM Corp., Armonk, NY, USA). The Crosstabs was used to assess categorical variables between patients with and without PSCI. Demographic was reported as number and percentage. An independent student t-test was used to calculate numerical variables and compare patients with and without PSCI. An independent samples test was performed to test the significance of plasma biochemical parameters. The level of statistical significance for all statistical analyses was set at P<0.05. Furthermore, forward stepwise logistic regression analysis was used to estimate the relative risk [odds ratio (OR) with a 95% confidence interval (CI)]. A receiver operating characteristic (ROC) curve was used to assess the sensitivity and specificity of independent risk factors in predicting PSCI.
ResultsOther Section
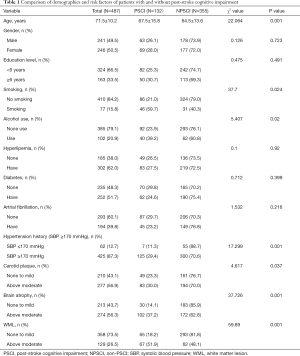
In total, 487 consecutive patients were included in the study. Among these patients, 132 (27.1%) patients were diagnosed as PSCI based on the criteria of DSM-IV. Age was related to PSCI (Table 1). In the statistical analysis, age ≥70 years was statistically significant (P=0.001). There was no significant difference in age between patients with 60 and 69 years. Another notable factor was systolic blood pressure history (SBP). Although there was no significant difference when the threshold of SBP was set to ≥140 and <170 mmHg, there were significant differences between the PSCI and non-PSCI groups when ≥170 mmHg was used as the threshold.
Full table
In addition, smoking (P=0.024) and alcohol consumption (P=0.02), above moderate cerebral atrophy (P=0.001), above moderate WML (P=0.001), and above moderate carotid plaque (P=0.032) were significantly more prevalent in PSCI patients than those without PSCI (Table 1). However, there was no difference between the two groups for other factors such as education, gender, hyperlipidemia, diabetes, or atrial fibrillation (P>0.05).
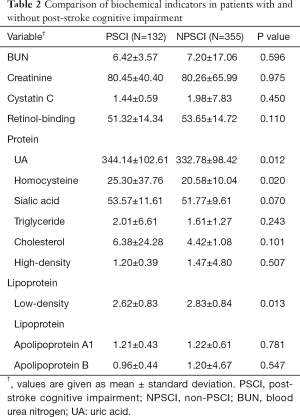
We then performed plasma biochemical parameters analysis to compare patients with PSCI and without PSCI with respect to creatinine, blood urea nitrogen (BUN), retinol-binding protein, uric acid (UA), cystatin C, sialic acid, triglyceride, cholesterol, high density lipoprotein, apolipoprotein A1, apolipoprotein B, homocysteine and low-density lipoprotein (LDL). We included a total of 13 factors in the evaluation (Table 2). The level of homocysteine (HCY), LDL, and UA between PSCI and non-PSCI patients were significantly different (P<0.05). However, no differences were identified for the other biochemical indicators (P>0.05).
Full table
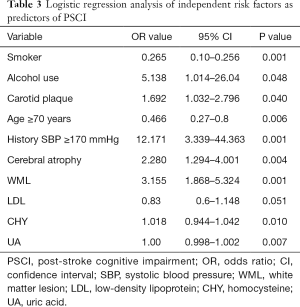
Logistic regression analysis found that multiple variables including alcohol use (OR: 5.138, 95% CI: 1.014–26.04, P=0.048), history of high systolic blood pressure (OR: 12.171, 95% CI: 3.339–44.363, P=0.001), carotid plaque (OR: 1.692, 95% CI: 1.032–2.796, P=0.040), cerebral atrophy (OR: 2.280, 95% CI: 1.294–4.001, P=0.004), and WML (OR: 3.155, 95% CI: 1.868–5.324, P=0.001) were all independently and significantly associated with PSCI (Table 3). Logistic regression also showed that homocysteine (OR: 1.018, 95% CI: 0.944–1.042, P=0.010) and UA (OR: 1.00, 95% CI: 0.998–1.002, P=0.007) and LDL (OR: 0.83, 95% CI: 0.6–1.148, P=0.051) significantly predicted PSCI development.
Full table
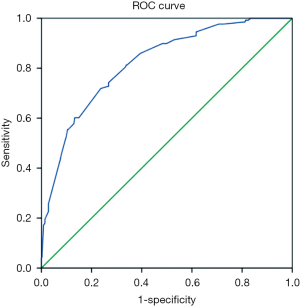
ROC curve analysis of the sensitivity and specificity of PSCI prediction found that the area under the curve (AUC) was 0.821 (95% CI: 0.78–0.862) (Figure 1). AUC had a certain accuracy at 0.7–0.9 and higher accuracy at 0.9 or above.
DiscussionOther Section
This study revealed that older age and the presence of carotid plaque, cerebral atrophy, above moderate WML, alcohol use, and smoking were all highly associated with the development of PSCI.
A number of studies have shown that having a stroke is highly correlated with cognitive impairment, which all share common risk factors (9). A report by Sibolt et al. (10) highlighted that an ischemic stroke is a risk factor for cognitive impairment and dementia; while, there are many other studies that have confirmed that PSD is associated with the recurrence of an ischemic stroke (11,12). One study found that the risk of incidents of dementia increased 4 folds among ischemic stroke patients relative to clinically stroke-free elderly control participants (13).
During the research, our data showed that age is related to PSCI occurrence. Age is one of the independent risk factors for PSCI. These results indicate that older patients are more prone to vascular cognitive impairment. In addition, our results revealed a relationship between alcohol consumption and cognitive impairment. Chronic alcohol consumption induces damage to the brain that can cause various forms of dementia. An abundance of acetaldehyde is produced by excessive alcohol consumption and accumulates in the body to induce oxidative stress, apoptosis, and inflammation in neuronal cells, which results in learning and cognitive decline (14). The presence of WML may be another predictor that is closely related to PSCI. In support of these findings, a review by Ihara (15) indicated that the presence and severity of WMLs measured by MRI might predict PSD. The authors also suggested that microstructural changes of normal-appearing white matter (WM), as evident in diffusion tensor imaging, maybe a good predictor of cognitive decline in patients with WM hyperintensities. A follow-up study of SMART-MR (16) found that cerebral atrophy, in combination with vascular brain lesions (WML and infarcts), was associated with an accelerated decline of executive functioning and memory. Our logistic regression analysis revealed that moderate and above brain atrophy is highly correlated with PSCI, which is consistent with the results of the above previous studies.
Our results, however, are not in agreement with the other reports on the relationship between hypertension and PSCI. A recent review (17) confirmed the close association of hypertension with having a stroke and reported there to be a relationship with cognitive impairment and dementia. From the findings of the previous reports, it can be concluded that arterial hypertension is likely the strongest risk factor for the different forms of VaD (18-20). On the contrary, Tamam et al. (21) argued that PSD did not significantly correlate with hypertension. Our study showed there was a significant difference between patients with and without PSCI in terms of a history of systolic blood pressure ≥170 mmHg. We found that 60% of patients with PSCI had a history of hypertension, and about 75% had a history of systolic blood pressure ≥170 mmHg. Yet there was no statistically significant difference between the two patient groups for a history of systolic blood pressure between 140 and 170 mmHg. Hypertension is one of the most important risk factors for atherosclerosis, which causes a stroke. Cerebral ischemia and hypoxia caused by stroke lead to neuronal damage and vascular cognitive impairment.
We extended our exploration to 13 plasma biochemical indicators for early prediction of PSCI and found significant differences in plasma HCY, LDL, and UA levels between patients with and without PSCI. HCY is a thiol-containing non-essential amino acid produced in all cells, as a product of normal folate and methionine metabolism. Elevated plasma homocysteine leads to hyperhomocysteinemia (HHCy). HHCy is a robust and independent risk factor for stroke and cognitive impairment (22-24). In the early stage of PSCI, cognitive function may be impaired by HHCY through: (I) pathway of vascular damage dependent on HCY; (II) disorder of lipid metabolism and promoting atherosclerosis; (III) promoting neuronal apoptosis; (IV) promoting neuronal development toxic effects of fenvalerate; (V) effects on nerve conduction and other mechanisms of cognitive function (25).
LDL transports all lipid molecules to the cells via the blood. LDL can be involved in the progression of atherosclerosis if these lipoproteins become oxidized within the artery walls. The formation of superoxide and free radicals causes oxidative damage to blood vessel walls, leading to thrombosis and the occurrence of ischemic cerebral infarction. Li et al. (26) demonstrated 87% sensitivity of LDL oxidation in the detection of VaD, which may serve as a novel biomarker to distinguish VaD and AD. A recent study in an elderly Brazilian population demonstrated that VLDL was associated with dementia as well as cognitive impairment without dementia (27).
Uric acid (UA) is the product of purine metabolism, which is a powerful antioxidant that may have neuroprotective properties, yet it is also a risk factor of vascular disease that predisposes individuals to cognitive impairment. In this study, we have shown that there is a positive association between serum UA concentrations and cognitive impairment. UA could stimulate vascular smooth muscle cell proliferation and induce endothelial dysfunction by inhibiting nitric oxide production. Elevated serum UA levels are also associated with systemic inflammation. These pathological changes also cause brain damage (28). Therefore, elevated plasma UA level is a potent risk factor for the development of cognitive impairment, which is consistent with many studies (28-30). Suzuki et al. reported (28) that elevated serum UA levels and cognitive deterioration remained statistically significant even after adjusting for confounding factors such as age, sex, BMI, education, SBP, fasting blood sugar, triglyceride, HDL-cholesterol, LDL-cholesterol, and serum creatinine levels. Therefore, the results of our study are of great significance.
Recently, the BMJ sub-journal (31) published a study on the dementia risk prediction model, which included factors such as age, sex, education level, cholesterol, alcohol consumption, and MMSE. Most of our research factors were included in this model. As the sample size and follow-up time were different between our study and the BMJ study, the prediction accuracy also varied. The AUC ranged from 0.63 to 0.92 in the BMJ study, while our ROC curve was 0.821. Our results show that the careful consideration of these factors will have better accuracy in predicting PSCI. And these indicators are routine diagnostic and therapeutic indicators not harmful to patients.
In conclusion, the variables such as drinking, cerebral atrophy, systolic blood pressure history, WML, carotid plaque between patients with PSCI, and without PSCI were significantly different while HCY, LDL, and UA may be risk factors of PSCI, which may be useful in the prediction of early dementia. Early identification of these factors will undoubtedly contribute to the prevention of PSCI. Therefore, further exploration of additional biomarkers warrants to understand the significance of these factors in PSCI better.
AcknowledgmentsOther Section
Thanks for all the members of the neuroscience research team of Bengbu First People’s Hospital.
FootnoteOther Section
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ReferencesOther Section
- Bordet R, Ihl R, Korczyn AD, et al. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: a consensus report. BMC Medicine 2017;15:107. [Crossref] [PubMed]
- Brainin M, Tuomilehto J, Heiss WD, et al. Post-stroke cognitive decline: an update and perspectives for clinical research. Eur J Neurol 2015;22:229-38. [Crossref] [PubMed]
- Mijajlović MD, Pavlović A, Brainin M, et al. Post-stroke dementia -a comprehensive review. BMC Medicine 2017;15:11. [Crossref] [PubMed]
- Viswanathan A, Macklin EA, Betensky R, et al. The influence of vascular risk factors and stroke on cognition in late life: analysis of the NACC cohort. Alzheimer Dis Assoc Disord 2015;29:287-93. [Crossref] [PubMed]
- Tang EYH, Robinson L, Stephan BCM. Risk prediction models for post-stroke dementia. Geriatrics (Basel) 2017. doi: 10.3390/geriatrics2030019. [Crossref]
- Yang J, Wong A, Wang Z, et al. Risk factors for incident dementia after stroke and transient ischemic attack. Alzheimers Dement 2015;11:16-23. [Crossref] [PubMed]
- Pantoni L, Basile AM, Pracucci G, et al. Impact of age-related cerebral white matter changes on the transition to disability-the LADIS study: rationale design and methodology. Neuroepidemiology 2005;24:51-62. [Crossref] [PubMed]
- Utter S, Tamboli IY, Walter J, et al. Cerebral small vessel disease-induced apolipoprotein E leakage is associated with Alzheimer disease and the accumulation of amyloid beta-protein in perivascular astrocytes. J Neuropathol Exp Neurol 2008;67:842-56. [Crossref] [PubMed]
- Rostamian S, Mahinrad S, Stijnen T, et al. Cognitive impairment and risk of stroke: a systematic review and meta analysis of prospective cohort studies. Stroke 2014;45:1342-8. [Crossref] [PubMed]
- Sibolt G, Curtze S, Melkas S, et al. Poststroke dementia is associated with recurrent ischaemic stroke. J Neurol Neurosurg Psychiatry 2013;84:722-6. [Crossref] [PubMed]
- Portegies ML, Wolters FJ, Hofman A, et al. Prestroke Vascular Pathology and the Risk of Recurrent stroke and Poststoke Dementia. Stroke 2016;47:2119-22. [Crossref] [PubMed]
- Moroney JT, Bagiella E, Tatemichi TK, et al. Dementia after stroke increases the risk of long-term stroke recurrence. Neurology 1997;48:1317-25. [Crossref] [PubMed]
- Pendlebury ST. Dementia in patients hospitalized with stroke: rates, time course, and clinico-pathologica factors. Int J Stroke 2012;7:570-81. [Crossref] [PubMed]
- Choi SH, Lee AY, Park CH, et al. Protective effect of Carthamus tinctorius L. seed on oxidative stress and cognitive impairment induced by chronic alcohol consumption in mice. Food Sci Biotechnol 2018;27:1475-84. [Crossref] [PubMed]
- Ihara M, Kalaria RN. Understanding and preventing the development of post-stroke dementia. Expert Rev Neurother 2014;14:1067-77. [Crossref] [PubMed]
- Kliper E, Ben Assayag E, Tarrasch R, et al. Cognitive state following stroke: the predominant role of preexisting white matter lesions. PLoS One 2014;9:e105461. [Crossref] [PubMed]
- Perrotta M, Lembo G, Carnevale D. Hypertension and Dementia: Epidemiological and Experimental Evidence Revealing a Detrimental Relationship. Int J Mol Sci 2016;17:347. [Crossref] [PubMed]
- Bath PM, Scutt P, Blackburn DJ, et al. Intensive versus guideline blood pressure and lipid lowering in patients with previous stroke: Main results from the pilot 'prevention of decline in cognition after stroke trial' (podcast) randomised controlled trial. PLoS One 2017;12:e0164608. [Crossref] [PubMed]
- Matz K, Teuschl Y, Firlinger B, et al. Multidomain lifestyle interventions for the prevention of cognitive decline after ischemic stroke: Randomized trial. Stroke 2015;46:2874-80. [Crossref] [PubMed]
- Strandgaard S, Paulson OB. Cerebrovascular consequences of hypertension. Lancet 1994;344:519-21. [Crossref] [PubMed]
- Tamam B, Taşdemir N, Tamam Y. The Prevalence of Dementia Three Months after Stroke and its Risk Factors. Turk Psikiyatri Derg 2008;19:46-56. [PubMed]
- Jayanthi N, Ayakrishnan SJ. Homocysteine: a biomarker in neurodegenerative diseases. Int J Biol Med Res 2015;6:5272-4.
- Dam K, Füchtemeier M, Farr TD, et al. Increased homocysteine levels impair reference memory and reduce cortical levels of acetylcholine in a mouse model of vascular cognitive impairment. Behav Brain Res 2017;321:201-8. [Crossref] [PubMed]
- Codoñer-Franch P, Alonso-Iglesias E. Homocysteine as a Biomarker in Vascular Disease. Biomarkers in Cardiovascular Disease 2016;381-406.
- Li H. Advances in hematological markers of vascular cognitive impairment. J Apoplexy and Nervous Diseases 2012;29:858-61.
- Li L, Willets RS, Polidori MC, et al. Oxidative LDL modification is increased in vascular dementia and is inversely associated with cognitive performance. Free Radic Res 2010;44:241-8. [Crossref] [PubMed]
- Lara VP, Caramelli P, Teixeira AL, et al. Cortisol, HDL-C,VLDL-c, and APOE polymorphisms as Laboratorial Parameters Associated to Cognitive impairment No Dementia (CIND) AND Dementia. J Clin Lab Anal 2016;30:374-80. [Crossref] [PubMed]
- Suzuki K, Koide D, Fujii K, et al. Elevated serum UA levels Are related to cognitive deterioration in an elderly Japanese population. Dement Geriatr Cogn Dis Extra 2016;6:580-8. [Crossref] [PubMed]
- Li Y, Gao M, Zhang Z, et al. Study on Cognitive Impairment serum uric acid and mild vascular disease in patients with cerebrovascular disease. Chin J Stroke 2018;13:237-41.
- Khan AA, Quinn TJ, Hewitt J, et al. Serum uric acid level and association with cognitive impairment and dementia: systematic review and meta-analysis. AGE 2016;38:16. [Crossref] [PubMed]
- Hou XH, Feng L, Zhang C, et al. Models for predicting risk of dementia: a systematic review. J Neurol Neurosurg Psychiatry 2019;90:373-9. [Crossref] [PubMed]


