
Effect of gonadotropin-releasing hormone analog on ovarian reserve in children with central precocious puberty
Introduction
The frequency and onset of precocious puberty (PP) rises annually over recent years, with changes in diets and living conditions. PP not only influences adult children’s height but may also be linked with adulthood diabetes, hypertension and some adult malignancies. In some situations, PP may contribute to psychosocial problems (1) and even crime contributing to mental, physical, and financial pressures on pediatric patients, parents, and the entire society.
Based on the activation of the hypothalamic-pituitary-gonadal axis (HPGA), two types of PP are recognized: central precocious puberty (CPP) and peripheral precocious puberty (PPP) (2). The prevalence of CPP is 10 times higher in girls than in boys (3), and most cases are idiopathic.
Standard treatment of CPP is based on postponement of skeletal maturation by blockade of pubertal development, so as to improve the final height in adulthood, help children and their families handle stress, and restore the psychological behaviors consistent with the chronological age of the children; also, these efforts may relieve employment pressure and prevent the occurrence of psychosocial problems. Gonadotropin-releasing hormone analog (GnRHa), including leuprorelin and triptorelin, is the mainstream treatment for CPP.
Many studies (4-6) have demonstrated that GnRHa treatment can effectively increase the final height of CPP girls in adulthood; however, few pieces of literature have investigated whether GnRHa treatment will ultimately affect the children’s reproductive function and whether the early prediction of children’s reproductive function is possible. It is currently believed that ovarian reserve is a good indicator of female reproductive function. It mainly refers to the ability of primordial follicles in the ovarian cortex to develop into fertilizable oocytes and is assessed in terms of the number of the follicles remaining in the ovary and the quality of ovum (7). At present, the commonly used measures of ovarian reserve include serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), FSH/LH, estradiol (E2), progesterone (P), inhibin B (INH B), anti-Müllerian hormone (AMH), uterine volume (Vuv), antral follicle count (AFC), ovarian volume (Vov), mean ovarian diameter, and ovarian stromal blood flow (8,9). The diagnosis criteria of diminished ovarian reserve (DOR) include: (I) a basal FSH level of >12 IU/L suggests decreased ovarian reserve (10); (II) FSH/LH >3 is used as a marker of declined ovarian reserve (11); (III) normal FSH level but elevated basal E2 level (>60–80 pg/mL) in the early follicular phase suggests a decrease in ovarian reserve (12); (IV) optimal reproductive function: serum AMH increases from birth up to 18 years, within the range of 4–6.8 ng/mL; suboptimal reproductive function: serum AMH level is 2.2–4 ng/mL (12); and poor ovarian response: AMH is <1.1 ng/mL (13); (V) Vuv and Vov: it is believed Vuv should be <3.0 mL and Vov <2.0 mL in prepubertal ages. Girls are entering puberty when unilateral Vov is ≥1–3 mL, along with 4 or more follicles sized ≥4 mm, and uterine length is >3.4–4.0 cm (14).
AMH is the earliest substance produced in follicles that can be detected in peripheral blood, and its change is earlier than the changes of other blood biomarkers; thus, it allows the prediction of changes in ovarian reserve in an earlier and more accurate manner (15,16). Many studies have reported that AMH is less affected by other factors, and its secretion is relatively stable, mainly changing with age. With higher specificity and sensitivity than other indicators, AMH has become a reliable marker for ovarian reserve (16-19).
This study was designed to retrospectively collect clinical data including serum LH, FSH, LH/FSH, E2, and AMH levels, Vov, Vuv, and GnRHa dose at various time points from CPP girls who had received GnRHa treatment for more than half a year in our hospital, with an attempt to analyze the effect of GnRHa treatment on the ovary reserve in CCP girls, predict the effect of GnRHa treatment on the reproductive function, eliminate the concerns of girls and their parents on the potential toxicities, improve the patients’ adherence to treatment, and thus achieve the goal of increasing the final height in the adulthood.
Methods
Subjects
A total of 383 CPP girls received GnRHa treatment in our center for at least 6 months during the period between September 2009 and February 2019, of which 383 were treated with triptorelin, 130 with leuprolide. Follow-up was conducted in 221 patients who had discontinued the medical treatment. The inclusion criteria were patients who met the diagnostic criteria for CPP and received GnRHa treatment. The exclusion criteria included: (I) boys; (II) pediatric patients with lesions or tumors in the thyroid, adrenal gland, and/or other organs; and (III) patients who had refused to receive GnRHa treatment. The study protocol was reviewed and approved by the Institutional Review Board of Jiangsu Subei People’s Hospital. Informed consents were waived by the board because the data in this study were collected retrospectively.
Methodology
Treatment algorithm
The initial dose of GnRHa treatment was 80–100 µg/kg administered every 4 weeks (maximum 3.75 mg). A simple GnRH stimulation test was performed 3 months later, during which the patient was injected with a GnRHa at the treatment dose, followed by the measurement of blood levels of LH, FSH, E2, and AMH 60 min later to learn the degree of HPGA suppression. Typically, an LH level of <2 U/L and an LH/FSH ratio of <1 indicate that the HPGA is suppressed in the child (20). The dose of GnRHa has adjusted accordingly.
Follow-up
The height, weight, secondary sexual characteristics, serum hormone (LH, FSH, E2, and AMH) levels, and Vuv and Vov were measured or observed every 3 months during the treatment, and the bone age was examined every 6 months. Serum hormone levels, changes in Vov and Vuv, and age of menarche or resumption of menses (ROM) were recorded after discontinuation.
Collection of clinical data
The clinical data including serum hormone (LH, FSH, E2, and AMH) levels, ovarian and uterine volumes, GnRHa dose, and course of treatment at baseline, after 6, 12, 18, 24, 30, 36, 42, and 48 months of treatment, and after menarche or ROM were retrospectively collected. The serum levels of LH, FSH, E2, and AMH were analyzed with a Roche commercial assay kit on Cobas® e601 (Roche Diagnostics, Mannheim, Germany) immune-analyzer using enzyme chemiluminescence immunoassay technique. The lowest detection limit was 0.1 IU/L for LH and 18.35 pmol/L for E2. Mindray Resona7S color ultrasound system was used to measure the length, transverse diameters, and anteroposterior diameters of the uterus and ovary. The volume (mL) was calculated by using the following formula: length (cm) × transverse diameter (cm) × anteroposterior diameter (cm) ×0.5233. The assessment of bone age was based on the Tanner and Whitehouse method (TW3).
Statistical analysis
Data is expressed as mean ± standard deviations (
Results
Baseline ovarian reserve before treatment
This research contained 383 subjects altogether. With AMH ≥1.1 ng/mL as the standard for normal ovarian reserve, 109 of the 117 CPP girls with baseline AMH levels available had a basal AMH level of ≥1.1 ng/mL, and thus 93% (109/117) of the patients were considered to have normal ovarian reserve at pre-treatment; with FSH/LH ≤3 as the standard for normal ovarian reserve, 122 of 265 CPP girls with basal FSH/LH ratio available had an FSH/LH ratio of ≤3, and therefore 46% (122/265) of the patients were considered to have normal ovary reserve at pre-treatment; with the basal FSH ≤12 IU/L as the standard for normal ovarian reserve, 262 of 265 CPP girls with basal FSH levels available had a basal FSH level of ≤12 IU/L, and therefore 99% (262/265) of the patients were considered to have normal ovary reserve at pre-treatment.
Effect of GnRHa on ovarian reserve during the treatment
Effect on Vuv
All the average Vuvs of CPP girls at different time points of GnRHa treatment were below the standard line (3 mL), and the Vuv gradually decreased over the treatment period, suggesting that GnRHa treatment was highly effective to arrest the uterine development at the prepubertal period. The Vuv was notably larger than 3ml after menarche and was greater than that before treatment, indicating the inhibitory effect of GnRHa treatment was reversible, and the ovarian reserve was not affected (Figure 1).

Effect on Vov
A Vov of <2 mL indicates that the hypothalamic-pituitary-adrenal axis (HPGA) function has not been activated, and the girls are still in the prepubertal period. In our current study, the average Vov at each time point during GnRHa treatment was below 2 mL for all CPP girls, indicating that GnRHa treatment was effective in arresting ovarian development in the prepubertal period. The Vov was notably larger than the standard line (2 mL) after menarche and was greater than that before treatment, indicating the inhibitory effect of GnRHa treatment was reversible after the girls entered the puberty and the ovarian reserve was not affected (Figure 2).

Effect on AMH
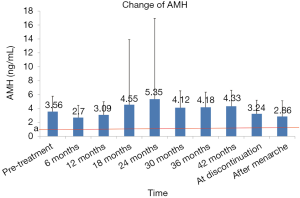
There was a transient decrease in AMH after initiation of GnRHa therapy. The AMH level was significantly lower after 6 months of treatment than pre-treatment (2.70±1.76 vs. 3.56±2.21); however, such inhibitory effect gradually disappeared as the treatment continued, as the AMH levels after 12, 18, and 24 months of treatment were similar to the pre-treatment level (all P>0.05). According to the literature (13), and AMH value below the standard line (1.1 ng/mL) indicates a poor ovarian response. All the AMH levels were above 1.1 ng/mL throughout the treatment despite some disparities at certain time points, indicating that the ovarian response did not decrease, and the ovarian reserve was not notably affected (Figure 3).
Effect on LH
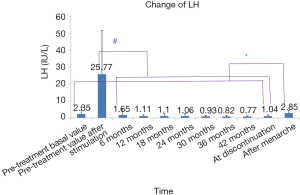
Since the LH value measured after menarche is the baseline value, the pre-treatment basal LV level was used for comparisons. All the LH levels (measured after a simple stimulation test) throughout the treatment were lower than the pre-treatment peak value, and the LH level showed a further decline as the treatment continued, indicating that the HPGA was well suppressed. The LH level after menarche was higher than those detected at other time points and increased compared with the pre-treatment basal LH level, suggesting that the suppression was reversible, and the treatment had little effect on the ovarian reserve (Figure 4).
Effect on FSH
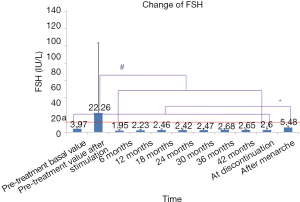
Since the FSH value measured after menarche is the baseline value, the pre-treatment basal LV level was used for comparisons. All levels of FSH (measured after a simple stimulation test) during treatment were lower than the peak value of pretreatment, suggesting that HPGA was well suppressed. The FSH level after menarche was higher than those detected at other time points during the treatment and increased compared with the pre-treatment basal FSH level, suggesting that the suppression of HPGA was reversible. According to the literature (10), and the FSH level of below 12 IU/L (the standard line) indicates that there is no decrease or reduction in ovarian reserve. Since all mean FSH levels (except the level calculated after pretreatment stimulation) were below the standard line throughout the treatment, it could be fairly concluded that GnRHa treatment had no effect on ovarian reserve (Figure 5).
Effect on FSH/LH value
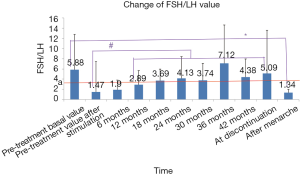
According to the literature (11), and FSH/LH value of >3 indicates a decreased ovarian reserve. In our current study, FSH/LH was significantly increased after 12 months (or longer) of GnRHa treatment when compared with the pre-treatment value after stimulation. The mean values of FSH/LH after 18 months of treatment were above 3, the standard line, suggesting that GnRHa treatment might cause certain damage to ovarian reserve. The mean value of FSH/LH at menarche was below the standard line 3 and lower than the pre-treatment basal value, indicating that such possible damage was reversible (Figure 6).
Effect on E2
All the mean values of E2 at each time point during treatment were lower than that before treatment, indicating that the ovary function was well suppressed. The mean value of E2 increased after menarche and exceeded the pre-treatment level, suggesting the suppression was reversible and had no adverse effect on ovarian reserve. According to the literature (12), a normal FSH level but elevated basal E2 level (>60–80 pg/mL) in the early follicular phase suggests a decrease in ovarian reserve (12). As shown in Figure 5, the FSH levels were within the normal range throughout the treatment. In our current study, the lowest standard line was an E2 level of 60 pg/mL (about 220.2 pmol/L). All the mean values of E2 were below the standard line throughout the treatment, indicating that the ovarian reserve was not affected (Figure 7).

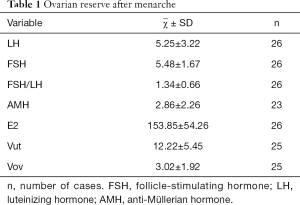
Ovarian reserve after menarche
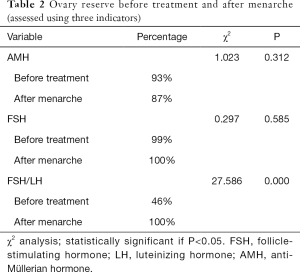
Up to now, medical treatment has been discontinued in 221 girls, among whom 123 have experienced menarche, as shown by telephone follow-up. The age of menarche was 11.93±1.38 years (range: 10.55–71 years) in these girls. The menstrual periods were regular in 81.3% (100/123) of girls within 2 years after menarche. Some children had poor medication compliance and did not participate in the follow-up visits; as a result, fewer data on biochemical blood indicators and ultrasound results are obtained. Finally, a total of 26 children with data after menarche entered the subgroup analysis (Table 1). For girls reaching menarche, an AMH of ≥1.1 ng/mL is the standard for normal ovarian reserve. In our current study, 20 (87%) of 23 CPP girls with AMH values available after menarche had an AMH level of ≥1.1 ng/mL and thus was considered normal ovary reserves; with FSH/LH ≤3 as the standard for normal ovarian reserve, 26 of 26 CPP girls with FSH/LH values available after menarche had an FSH/LH value of ≤3, and therefore 100% (26/26) of the patients were considered to have normal ovary reserve after menarche; with basal FSH ≤12 IU/L as the standard for normal ovarian reserve, 26 of 26 CPP girls with basal FSH values available after menarche had a basal FSH value of ≤12 IU/L, and therefore 100% (26/26) of the patients were considered to have normal ovary reserve after menarche. As shown by χ2 test (Table 2), comparison of ovarian reserve before and after treatment showed that the percentages of patients with normal FSH/LH and FSH level after GnRHa treatment were higher than those before treatment and the percentage of normal AMH was slightly lower than that before treatment, although the differences were not statistically significant, suggesting that the ovarian reserve after GnRHa treatment is non-inferior to that before treatment.
Full table
Full table
Discussion
GnRHa is currently the mainstream medical treatment for CPP (21) as it can down-regulate the receptors, suppress the function of the hypothalamic-pituitary-gonadal axis, reduce the secretion of E2, LH, and FSH, inhibit sexual development, postpone skeletal maturation, and thus increase final height. Annually, the number of girls diagnosed with CPP has increased as more girls start puberty earlier, and the use of GnRHa is also rising. Accordingly, the toxicities of long-term GnRHa treatment have become major concerns in these patients. The effectiveness of GnRHa treatment in improving the final height in adulthood has been well demonstrated (4-6). However, few studies have explored the effect of GnRHa treatment on the reproductive function of CPP girls. A sporadic report in the Republic of Korea found that GnRHa treatment had no adverse effect on the AMH level of CPP girls (22), while no similar research has been carried out in China. CPP girls are young, and their reproductive function must be studied only when they grow up, which takes a long period and can not eliminate the concerns of parents. In contrast, the ovarian reserve represents the reproductive ability of women. Therefore, early use of routine clinical indicators to explore the effect of GnRHa treatment on ovarian reserve in CPP girls is of great significance for increasing the treatment compliance and improving the prognosis of CPP girls. In our current study, we followed up and statistically analyzed the relevant indicators of ovarian reserve in CPP girls treated with GnRHa.
These currently used indicators include Vuv, Vov, LH, FSH, FSH/LH, E2, AMH, AFC, and inhibin B (INHB) as well as the results of exogenous ovarian stimulation test and ovarian biopsy (8,9). As any single indicator has its disadvantages, multiple indicators were measured in our current study to improve the accuracy and reliability of the assessment. Because some tests are invasive and/or limited by detection techniques, we measured (or calculated) Vuv, Vov, LH, FSH, FSH/LH, E2, and AMH to assess the ovarian reserve in CPP girls.
Vuv, LH, FSH, and E2
The serum levels of Vuv, LH, FSH, and E2 always points after the initiation of GnRHa treatment were decreased compared with a pre-treatment. During treatment, the pituitary function was inhibited, and the decreased LH and FSH secretions led to the reduction in the downstream hormone E2, which further resulted in the shrinkage of the uterus and ovary. The levels of Vuv, LH, FSH, and E2 remarkably increased at menarche after discontinuation, indicating that the inhibitory effect of GnRHa on HPGA is reversible and the ovarian reserve is not affected.
AMH
As reported in the literature (12), and AMH level of <1.1 ng/mL suggests poor ovarian response (POR). In our current study, the AMH level showed a transient decrease after 6 months of GnRHa treatment; however, such inhibitory effect gradually disappeared as the treatment continued, as the AMH levels after 12, 18, and 24 months of treatment were similar to the pre-treatment level. All the AMH levels were above 1.1 ng/mL throughout the treatment despite some disparities at certain time points, indicating that the ovarian response did not decrease, and the ovarian reserve was not notably affected. This finding was consistent with the result of a foreign study (22).
FSH/LH
According to literature, the ovarian reserve begins to decline when FSH/LH is >3 (11). In our current study, FSH/LH was significantly increased after 12 months (or longer) of GnRHa treatment when compared with the pre-treatment value after stimulation. The mean values of FSH/LH after 18 months of treatment were above standard line 3, suggesting that GnRHa treatment might cause certain damage to ovarian reserve. The mean value of FSH/LH at menarche was below the standard line 3 and lower than the pre-treatment basal value, indicating that such possible damage was reversible and the effect of GnRHa treatment on the ovarian reserve was small after discontinuation.
Ovarian reserve before and after treatment
Comparison of ovarian reserve before and after treatment showed that the percentages of patients with normal FSH/LH and FSH level after GnRHa treatment were higher than those before treatment and the proportion of normal AMH was slightly lower than that before treatment, although the differences were not statistically significant, suggesting that the ovarian reserve after GnRHa treatment is non-inferior to that before treatment. All the above standards are for adults, however, and whether they can be applied to children remains unclear. Therefore, when the criteria of FSH/LH ≤3 was applied, only 46% of the CPP girls had an abnormal ovarian reserve. Although the HPGA had been initiated in these girls, it was not yet mature, and the FSH/LH value could not reach the adult standard; thus, it could not be concluded that the ovarian reserve was low. In addition, the Vut and Vov criteria were based on foreign findings, and possible ethnic diversities should be taken into account.
Age of menarche
As shown in our follow-up visits, the age of menarche was 11.93±1.38 years (range: 10.55–13.31 years) in our series, similar to most normal girls. In addition, up to 81.3% of CPP girls had regular menstrual cycles. Healthy girls in the early years of puberty can also experience irregular periods, with anxiety and stress also causing regular periods in CPP girls. Similarly, a study has also shown that GnRHa-treated CPP girls had similar age of menarche to normal healthy girls, and their menstruation was regular (23). Therefore, GnRHa treatment has no adverse effect on ovarian reserve in CPP girls.
In summary, GnRHa treatment has similar effects on ovarian reserve markers including uterine and ovarian volumes, LH, FSH, and E2: these markers were inhibited during treatment (compared with the pre-treatment levels), gradually recovered after discontinuation, and became higher than the pre-treatment level levels after menarche, suggesting GnRHa has an inhibitory effect on ovarian reserve during the treatment, but such effect is reversible and restoring the ovarian function after menarche. AMH showed a transient decrease after 6 months of GnRHa treatment; however, such inhibitory effect gradually diminished after 12 months of treatment. All the AMH levels were above 1.1 ng/mL throughout the treatment, indicating that the ovarian response did not decrease, and the ovarian reserve was not notably affected. FSH/LH was significantly increased after GnRHa treatment when compared with the pre-treatment value after stimulation; the mean values of FSH/LH after 18 months of treatment were above the standard line 3, suggesting that GnRHa treatment might cause certain damage to ovarian reserve. The mean value of FSH/LH at menarche was below the standard line 3 and lower than the pre-treatment basal value, indicating that such possible damage was reversible and had no notable impact on the ovary reserve after discontinuation.
To sum up, the ovarian reserve of CPP girls is somehow inhibited during GnRHa treatment but is gradually restored after discontinuation and is non-inferior to the pre-treatment levels. Assessment of the changes in ovarian reserve indicators, including Vuv, Vov, LH, FSH, FSH/LH, E2, and AMH, allows the early evaluation of reproductive function in CPP girls, eliminate the parents’ concerns on GnRHa treatment and thus increase the treatment compliance.
Limitations and prospects
- First, due to the poor treatment compliance, the sample sizes of patients at different time points after discontinuation and after menarche were small, and thus the trend of changes at different time points from discontinuation to menarche could not be analyzed.
- Second, although AMH, AFC, FSH/LH, and inhibin B (InhB) are good indicators of ovarian reserve, no age-specific cut-off value has been available, and the application of the criteria for adults in CPP girls may be less persuasive.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was reviewed and approved by the Institutional Review Board of Jiangsu Subei People’s Hospital. Informed consents were waived by the board because the data in this study were collected retrospectively.
References
- Cheong JI, Lee CH, Park JH, et al. The effect of early menarche on the sexual behaviors of Korean female adolescents. Ann Pediatr Endocrinol Metab 2015;20:130-5. [Crossref] [PubMed]
- Endocrine Genetics Group of Chinese Medical Association Pediatrics Branch & Editorial Board of Chinese Journal of Pediatrics. Expert consensus on CPP diagnosis and treatment (2015). Chinese Journal of Pediatrics 2015;53:412-8. [PubMed]
- Ren ML, Zheng YN. Diagnosis and treatment strategies for female precocious puberty. Journal of Practical Obstetrics and Gynecology 2017;33:572-5.
- Chen QL. GnRHa improves the adult stature of girls with central precocious puberty and early puberty (rapidly progressive variety): a 15-year follow-up study of 102 cases in a single center A. in Chinese Medical Association, Chinese Medical Association Pediatrics Branch. Proceedings of Chinese Medical Association 17th National Pediatrics Conference (Volume 1) C. Chinese Medical Association, Chinese Medical Association Pediatrics Branch: Chinese Medical Association, 2012:2.
- Ying Y, Tang J, Chen W, et al. GnRH agonist treatment for idiopathic central precocious puberty can improve final adult height in Chinese girls. Oncotarget 2017;8:109061-7. [Crossref] [PubMed]
- Lee HS, Yoon JS, Park KJ, et al. Increased final adult height by gonadotropin-releasing hormone agonist in girls with idiopathic central precocious puberty. PLoS One 2018;13:e0201906. [Crossref] [PubMed]
- Kitajima M, Khan KN, Harada A, et al. Association between ovarian endometrioma and ovarian reserve. Front Biosci (Elite Ed) 2018;10:92-102. [Crossref] [PubMed]
- Tal R, Seifer DB. Ovarian reserve testing: a user's guide. Am J Obstet Gynecol 2017;217:129-40. [Crossref] [PubMed]
- Chen WJ, Li HF, Zhou BB, et al. Advances in evaluation and treatment of ovarian reserve. The Journal of Practical Medicine 2016;32:19-22.
- van der Steeg JW, Steures P, Marinus J, et al. Predictive value and clinical impact of basal follicle stimulating hormone in subfertile, ovulatory women. J Clin Endocrinol Metab 2007;92:2163-8. [Crossref] [PubMed]
- Kofinas JD, Elias RT. Follicle-stimulating hormone/luteinizing hormone ration as an independent predictor of response to con-trolled ovarian stimulation. Womens Health (Lond) 2014;10:505-9. [Crossref] [PubMed]
- Xie X, Kong BH, Duan T. Obstetrics and Gynecology. 9th edition. Beijing: People's Medical Publishing House, 2018.
- Cui L, Qin Y, Gao X, et al. Anti-Müllerian hormone: Correlation with age and androgenic and metabolic factors in women from birth to postmenopause. Fertil Steril 2016;105:481-5.e1. [Crossref] [PubMed]
- Ziereisen F, Guissard G, Damry N, et al. Sonographic imaging of the pediatric female pelvis. Eur Radiol 2005;15:1296-309. [Crossref] [PubMed]
- Zhou XY. Influencing factors of anti-Mullerian hormone detection results. Chinese and Foreign Medical Research 2018;16:183-5.
- Li XT, Du DL. Application of anti-Mullerian hormone in evaluating ovarian reserve. Journal of Bengbu Medical College 2018;43:1418-20.
- Yu J, Li Q, Tian XW, et al. Value of anti-Mullerian hormone combined with B-ultrasound in the prediction of ovarian reserve function. China Continuing Medical Education 2018;10:66-7.
- Mossa F, Ireland JJ. Physiology and endocrinology symposium: Anti-Müllerian hormone: a biomarker for the ovarian reserve, ovarian function, and reproductive function in dairy cows. J Anim Sci 2019;97:1446-55. [Crossref] [PubMed]
- La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update 2014;20:124-40. [Crossref] [PubMed]
- Kendirci HN, Ağladıoğlu SY, Baş VN, et al. Evaluating the Efficacy of Treatment with a GnRH Analogue in Patients with Central Precocious Puberty. Int J Endocrinol 2015;2015:247386.
- Hagen CP, Sørensen K, Anderson RA, et al. Serum levels of antimüllerian hormone in early maturing girls before, during, and after suppression with GnRH agonist. Fertil Steril 2012;98:1326-30. [Crossref] [PubMed]
- Nam HK, Kim HR, Rhie YJ, et al. Serum Anti-Müllerian Hormone Levels in Precocious Puberty Girls according to Stage of GnRH Agonist Treatment. J Korean Med Sci 2017;32:475-9. [Crossref] [PubMed]
- Baek JW, Nam HK, Jin D, et al. Age of menarche and near adult height after long-term gonadotropin-releasing hormone agonist treatment in girls with central precocious puberty. Ann Pediatr Endocrinol Metab 2014;19:27-31. [Crossref] [PubMed]


