
Protocol for the development of a rapid advice guidelines for management of children with SARS-CoV-2 infection
Introduction
In December 2019, a new type of Coronavirus that causes pneumonia was first detected in Wuhan, China, and is since then spreading worldwide (1). The virus was named SARS-CoV-2 later on, and the infectious disease was officially named “Corona Virus Disease 2019” (COVID-19) by World Health Organization (WHO) on February 11, 2020. Subsequently, WHO ranked the risk of a global SARS-CoV-2 outbreak to high, saying it poses a very high risk for China and the world (2). To date, over 30,000 cases have been diagnosed and 723 deaths have been confirmed. The virus has now reached several other countries. Two hundred and seventy-four cases had been diagnosed in 24 countries so far including Japan, South Korea, Singapore, UK and the United States (3). The WHO has declared that the new coronavirus outbreak is a public health emergency of international concern on 30 January 2020. Children COVID-19 cases, including adolescents, have also been reported, and their number seems to be on the increase. Current guidelines and recommendations mainly target adults with SARS-CoV-2 infection. Health care providers are in urgent need of a guideline to assist the diagnosis and management of SARS-COV-2 infected children. Rapid advice guidelines need to be completed in 1 to 3 months, and are designed primarily to respond to public health emergencies, in order to provide timely and rapid guidance to health workers (4). We aim to develop a rapid advice guideline using a multidisciplinary and collaborative approach. The guideline will follow the methods for developing WHO rapid advice guidelines (5), taking into account the special condition of this infectious disease, in order to respond to the health emergency, need for evidence-based guidance.
Methods
The guideline will be developed in accordance with the WHO requirements for rapid advice guideline (4), followed the new guideline definition from the Institute of Medicine (IOM) (6). Other related guidelines on SARS-CoV-2 will be evaluated by the AGREE II instrument (Appraisal of Guidelines for Research and Evaluation) before the guideline development (7). The guideline will meet the criteria of Guideline 2.0 and RIGHT (Reporting Items for Practice Guidelines in Healthcare) checklist of guideline development version 2.0 (8) and RIGHT statement (9). Gantt chart illustrates the key steps and timeline of the guideline (Figure S1).
Guideline developers
The China National Clinical Research Center for Children Health and Disease (Children's Hospital of Chongqing Medical University), Clinical Child Pharmacology Society of Pediatric Academy of Chinese Medical Association, National Clinical Research Center for Infectious Disease (Shenzhen Third People’s Hospital), G-I-N Asia, Chinese GRADE Center and WHO Collaborating Centre for Guideline Implementation and Knowledge Translation jointly initiated the development of the guideline.
Guideline registration
The guideline was registered at the International Practice Guidelines Registry Platform (http://www.guidelines-registry.org/). The registration No. is IPGRP- 2020CN008.
Guideline working groups and declaration of conflicts of interest
The guideline working group consists of a guideline development group and a rapid review group. All members of the guideline working group are required to make a conflicts of interest declaration (10). These declarations will be included as attachments with the final guideline document. The development of the guidelines is supported by a special fund from The China National Clinical Research Center for Children Health and Disease (Children’s Hospital of Chongqing Medical University), 2020 Key R & D project of Gansu Province and Prevention and Control of emergency of COVID-19 from Key Laboratory of Evidence Based Medicine and Knowledge Translation of Gansu Province (No. GSEBMKT-2020YJ01).
Guideline Development Group
The guideline development group will consist of 37 multidisciplinary experts, including infectious disease physicians, respiratory physicians, public health experts, clinical pharmacists, methodological experts, nurse practitioners, primary care pediatricians, general practitioners, legal experts and global health researchers. The main responsibilities of the development group are to draft proposal, prioritize clinical questions, participate in Delphi surveys, reach a consensus and approve the guideline. For each recommendation, if it receives more than two thirds of votes then the agreement will be reached.
Rapid Review Group
The rapid review group consists of methodologists and pediatricians. The main responsibilities of the rapid review group are to collect initial clinical questions, conduct rapid reviews, rate the evidence, prepare decision-making tables and take the minutes of guideline meeting.
Guideline scope
The title of the guideline is rapid advice guidelines for management of children with SARS-CoV-2 infection. The guideline is targeted for pediatricians, clinical pharmacists, general practitioners, and nurses in general hospitals, children’s hospitals, and primary clinics. The target population and beneficiary of the guideline are children, including adolescents (<18 years). The guideline will contain the following sections: new terms and definitions, symptoms, screening and diagnosis, risk assessment, lab testing and imaging tests, treatments, management, and patient education.
Determination of clinical questions
The clinical questions and outcomes will be collected and sorted out by the members of the rapid review group and sent to the panelist by emails. The development group will add, revise, prioritize and confirm the final set of clinical questions. The guideline is intended to include 10 to 20 clinical questions.
Evidence search
The rapid review group will search and evaluate the evidence. A search strategy will be developed and implemented to identify literature published since 2003 [considering the first novel coronavirus caused the severe acute respiratory syndrome (SARS) outbreak was in 2003] to February 2020. The following databases will be searched: PubMed, EMBASE, Cochrane library, Web of Science, WANFANG, CNKI, and CBM. There will be no restriction on publication type. Box S1 shows the PubMed database search as an example.

Full table
Evidence screening and data extraction
At least two reviewers will screen the titles, abstracts and full texts in accordance with the inclusion and exclusion criteria of each clinical question independently, then extract the data using a pre-designed data extraction form. In case of disagreements, consensus will be achieved with the help of an experienced third researcher.
Conduct and update systematic review
The rapid review group will first retrieve any existing relevant systematic reviews. If they meet our criteria (published within three years and have high quality evaluated by AMSTAR) (11), the group will adopt them. If they are of high quality but outdated, the group will update them. If they are of low quality, or they do not answer our PICO questions, the group will conduct a new rapid review.
Grade the quality of evidence
The rapid review group will use the GRADE system to rate the envidence (12,13). The evidence will be downgraded according to the following five criteria (risk of bias, inconsistency, indirectness, imprecision and publication bias) or upgraded according to the following three criteria (large magnitude of effect, dose response, confounders likely minimize the effect) (14-19). The final certainty of evidence will be graded as either high, moderate, low or very low (20).
Formulate recommendations and reach consensus
The guideline development group will propose an initial draft set of recommendations and use two rounds of a modified Delphi to reach a consensus by a GRADE Grid table (Table S1).
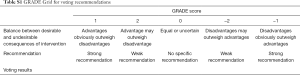
Full table
Discussion
Although health staff has been going through the new coronavirus infection diseases like the SARS and MERS for many years, they still lack high-quality guidelines to rely on when in the context of the emergency: SARS-CoV-2 outbreak. The first reason is effective antivirus medication may be limited and some agents probably have worrying side effects for the children. Some agents used in the clinic are controversial without high-quality evidence to support. Secondly, some unnecessary tests may increase the health care burden, but with no benefit of diagnosis. Moreover, for a fulminating infectious disease, the screening, and management strategy should be standard and efficient. Tremendous health care resources have been put in the control of the virus spread, and it should be used reasonably under a smart policy. There is no doubt that clinical practice guidelines can provide the best recommendations for clinicians based on evidence from systematic reviews (5).
The development of standard guidelines needs to follow a strict methodology and generally requires a period of more than one year. Thus, the time-consuming development has become a major concern for both guideline makers and users (21-22) . Therefore, the standard guideline process is not suitable for producing timely guidance and in response to public health emergencies such as the infectious disease SARS and COVID-19. Guideline developers have begun to explore more rapid approaches. WHO proposed the concept of rapid advice guidelines around 2006, which is to be developed over a period of one to three months to respond to the public health emergencies or other needs (23).
Rapid advice guidelines meet the minimum standards as described in the WHO guideline manual, with reasonably modified process and methods that allow to quickly complete the development of the guideline. Therefore, rapid advice guidelines, based on the available up-to-date and high-quality evidence, can help the healthcare providers make quick evidence-based decisions, and its development process is accelerated. WHO has developed ten rapid advice guidelines for the prevention of infectious diseases such as H5N1, HIV, tuberculosis, H1N1, Ebola, and Zika virus (24-29). So far two rapid advice guidelines have been developed in China (30,31). To our best knowledge, this is the first international rapid advice guideline on children with SARS-CoV-2 infection. The guideline will be made available in English, Japanese, Korean, and simplified and traditional Chinese. We believe the guideline will help policy makers, health practitioners and parents take better care of children with SARS-CoV-2 infection.
Acknowledgments
We are extremely thankful to Amir Qaseem, Detty Nurdiati, Edwin Chan, Hongmei Xu, Hyeong Sik Ahn, Janne Estill, Joseph Mathew, Junqiang Lei, Liqun Wu, Lei Liu, Mansuk Daniel Han, Mengshu Wang, Myeong Soo Lee, Qi Wang, Quan Lu, Ruiqiu Zhao, Rosalind Smyth, Shihui Liu, Shu Yang, Shunyin Zhao, Toshio Fukuoka, Wilson Milton Were, Wenwei Tu, Wong Wing Kin Gary, Xianlan Zheng, Xiaobo Zhang, Xiaodong Zhao, Xiaoping Luo, Xiaoxia Lu, Xixi Feng, Yuan Qian, Zhengxiu Luo, Zhihui He, and Zhou Fu for their comments and revisions on earlier drafts of the paper.
Funding: The project is funded by the following funding agencies: (I) The China National Clinical Research Center for Children Health and Disease (Children’s Hospital of Chongqing Medical University); (II) Chongqing technology innovation and application development special project fund; (III) 2020 Key R & D project of Gansu Province, China; (III) Prevention and Control of emergency of COVID-19 from Key Laboratory of Evidence Based Medicine and Knowledge Translation of Gansu Province (No. GSEBMKT-2020YJ01).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm.2020.02.33). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- World Health Organization. Surveillance case definitions for human infection with novel coronavirus (nCoV). 2020; WHO/2019-nCoV/Surveillance/v2020.2.
- National Health Commission of the People's Republic of China. Prevention and treatment of pneumonia caused by new coronavirus infection. 2020. Available online: http://www.nhc.gov.cn/
- Garritty CM, Norris SL, Moher D. Developing WHO rapid advice guidelines in the setting of a public health emergency. J Clin Epidemiol 2017;82:47-60. [Crossref] [PubMed]
- World Health Organization. WHO handbook for guideline development: WHO, 2014.
- Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Clinical Practice Guidelines We Can Trust. Washington (DC): National Academies Press (US); 2011.
- Brouwers MC, Kerkvliet K, Spithoff K, et al. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ 2016;352:i1152. [Crossref] [PubMed]
- Schünemann HJ, Wiercioch W, Etxeandia I, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ 2014;186:E123-42. [Crossref] [PubMed]
- Chen Y, Yang K, Marušić A, et al. A reporting tool for practice guidelines in health care: the RIGHT statement. Ann Intern Med 2017;166:128-32. [Crossref] [PubMed]
- Wang X, Chen Y, Yao L, et al. Reporting of declarations and conflicts of interest in WHO guidelines can be further improved. J Clin Epidemiol 2018;98:1-8. [Crossref] [PubMed]
- Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Norris SL, Meerpohl JJ, Akl EA, et al. The skills and experience of GRADE methodologists can be assessed with a simple tool. J Clin Epidemiol 2016;79:150-8.e1. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J Clin Epidemiol 2011;64:407-15. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence--publication bias. J Clin Epidemiol 2011;64:1277-82. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol 2011;64:1283-93. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol 2011;64:1294-302. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence--indirectness. J Clin Epidemiol 2011;64:1303-10. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 2011;64:1311-6. [Crossref] [PubMed]
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401-6. [Crossref] [PubMed]
- Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. Lancet 2017;390:415-23. [Crossref] [PubMed]
- Raine R, Sanderson C, Black N. Developing clinical guidelines: A challenge to current methods. BMJ 2005;331:631-3. [Crossref] [PubMed]
- Schünemann HJ, Hill SR, Kakad M, et al. Transparent development of the WHO rapid advice guidelines. PLoS Med 2007;4:e119. [Crossref] [PubMed]
- World Health Organization. WHO rapid advice guidelines on pharmacological management of humans infected with avian influenza A (H5N1) virus. Geneva: World Health Organization; 2006.
- World Health Organization. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. Geneva: World Health Organization; 2011.
- World Health Organization. Rapid advice: treatment of tuberculosis in children. Geneva: World Health Organization; 2010.
- World Health Organization. Clinical management of adult patients with complications of pandemic influenza A (H1N1) 2009: emergency guidelines for the management of patients with severe respiratory distress and shock in district hospitals in limited-resource settings. Geneva: World Health Organization; 2010.
- World Health Organization. Screening, assessment and management of neonates and infants with complications associated with Zika virus exposure in utero: rapid advice guideline. Geneva: World Health Organization; 2016.
- Lamontagne F, Fowler RA, Adhikari NK, et al. Evidence-based guidelines for supportive care of patients with Ebola virus disease. Lancet 2018;391:700-8. [Crossref] [PubMed]
- Endocrinology Society of Chinese Medical Association, Chinese Pharmaceutical Association Hospital Pharmacy Committee. the Quick Advice Guideline for the Clinical Application of Incretin-Based Drug Therapy. Chin J Endocrinol Metab 2016;32:448-54.
- Zhou P, Liang S, Zhai S. A Protocol Introduction of Rapid Advice Guideline for Intravenous Azithromycin in Pediatrics. China Pharm 2018;29:436-40.



