Adjuvant chemotherapy improves the prognosis of early stage resectable pulmonary large cell carcinoma: analysis of SEER data
Introduction
Large cell carcinoma (LCC) is a rare subtype of non-small cell lung cancer (NSCLC), with an incidence of about 10% in NSCLC (1,2). According to the 2004 WHO lung cancer classification (3), LCC belongs to the undifferentiated cancer with poor prognosis and has five different variants. It has been reported that LCC mainly occurs in the elderly and is closely related to the cigarette smoking (4,5). Moreover, the diagnosis of LCC is largely dependent on post-operative pathological examination (6,7). In addition, currently, there is no recommended treatment for LCC, thus its treatments are often chosen according to the treatment experience of NSCLC. And it has confirmed the survival benefits of adjuvant postoperative chemotherapy in stage II and IIIA NSCLC patients (8,9), but the value of adjuvant chemotherapy for stage IB in NSCLC remains controversial. In the Cancer and Leukemia Group B (CALGB) trial 9633, carboplatin-based adjuvant chemotherapy was found to have no influence on the prognosis of stage IB for NSCLC (10), which was also supported by the results of Park et al. (11) and Li et al. (12). Nevertheless, other studies had revealed opposite outcomes (13-15).
As a subtype of poor prognosis of NSCLC, the clinical characteristics, and treatments of early stage LCC are not well known. And the role of chemotherapy after surgery is still unclear in the early stage LCC. So in our study, the patients of LCC in the SEER database were retrospective reviewed, and the characteristics, prognosis, and survival of these patients were further analyzed.
Methods
Data extraction
Data of patients diagnosed with LCC between 2004 and 2015 were extracted using the SEER*Stat software version 8.3.5. The study cohort comprised patients who were diagnosed with LCC according to the International Classification of Disease for Oncology, the third edition (ICD-O-3) histology code 8012/3 (LCC, NOS), 8013/3 (large cell neuroendocrine carcinoma), and 8014/3 (LCC with rhabdoid phenotype). The large cell neuroendocrine carcinoma was also contained based on the 2004 WHO lung cancer classification (3). The exclusion criteria were as follows: (I) patients with more than one primary cancer; (II) patients diagnosed at stage III/IV; (III) patients without pathological confirmation based on histology; (IV) the clinical information was incomplete, including age, gender, race, marital status, primary site, laterality, grade, size, stage, chemotherapy, surgery and survival data. The TNM staging was reclassified for each patient based on the primary tumor size and tumor invasion according to the TNM classification for lung cancer (8th Edition) (16) using R version 3.4.3 software. Approval was waived by the local ethics committee, as SEER data is publicly available and de-identified.
Overall survival (OS) refers to the interval from the date of diagnosis to the date of death or of the last follow-up. The survival time less than 1 month (encoded as zero in the SEER database) was assigned to 0.5 months according to the standard epidemiological convention.
Statistical analysis
Categorical variables were analyzed with the Pearson χ2 test. The Kaplan-Meier method was used to estimate the survival probabilities with the log-rank test to assess any significant difference between OS stratified by each covariate. Cox proportional hazards model performed to assess the independent clinicopathological characteristics associated with the survival. Only the variables significantly related to the survival in univariate analysis were enrolled in multivariate analysis. Moreover, the nomogram was established based on the results of multivariate analysis by using R version 3.4.3 software. Prediction error was estimated with 1,000 bootstrap samples. A value of two-sided P<0.05 was considered statistically significant. Statistical analysis was conducted with SPSS version 25.0 (SPSS, Chicago, IL), and the GraphPad Prism 7 (GraphPad Software, San Diego, CA) was used to delineate the survival curve.
Results
Treatments of early-stage LCC
As shown in Table 1, a total of 1,099 patients with LCC were comprised in this study. 73.2% of patients underwent primary surgery alone, while the others received surgery with post-operative chemotherapy. Notably, 71.8% of patients were over 60 years old, and 66.7% of the lesions located in the upper lobe, followed by the lower lobe (25.7%). Most of tumors (96.1%) showed poor differentiation or undifferentiation. Furthermore, 64.1% of patients were diagnosed with LCC at stage I (IA: 38.3%; IB: 25.8%), and 35.9% at stage II. In addition, the age, tumor size, stage, and marital status were the factors related to the use of post-operative chemotherapy. Compared with patients with surgery alone, patients treated with adjuvant chemotherapy were younger (P<0.001), married cases (P=0.013), higher stage (P<0.001) and larger lesions (P<0.001).

Full table
OS of LCC patients
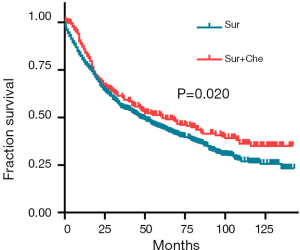
The median OS was 47 months (range, 0.5–143 months) in patients with surgery alone and 61 months (range, 0.5–142 months) in those with post-operative chemotherapy. The cases with post-operative chemotherapy had a better OS than those with surgery alone (HR: 0.805; 95% CI: 0.676–0.959, P=0.020) (Figure 1). The 1-, 3-, 5-year survival rate for patients with combined chemotherapy was 85.7%, 60.6%, 50.7, separately, compared with 79.4%, 55.1% and 44.6% in cases with surgery alone.
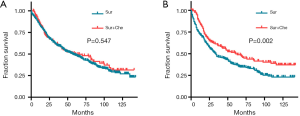
For the patients with tumor size ≤3 cm, there was no significant difference in the prognosis between patients with surgery alone and those with post-operative chemotherapy (HR: 0.918, 95% CI: 0.700–1.205, P=0.547) (Figure 2A). However, patients with post-operative chemotherapy had a better OS than those with surgery alone in patients with tumor size >3 cm (HR: 0.670, 95% CI: 0.528–0.851, P=0.002) (Figure 2B).
Meanwhile, the patients with stage IA LCC could not benefit from the post-operative chemotherapy in OS (HR: 0.966, 95% CI: 0.625–1.491, P=0.877, Figure 3A), but a significant difference was observed in OS between patients with or without post-operative chemotherapy in those with stage IB LCC (HR: 0.579, 95% CI: 0.411–0.815, P=0.006, Figure 3B) or stage II LCC (HR: 0.684, 95% CI: 0.529–0.883, P=0.004, Figure 3C).

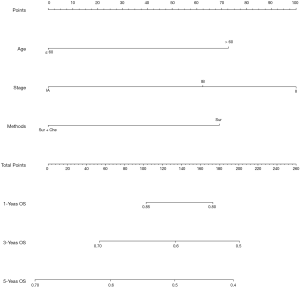
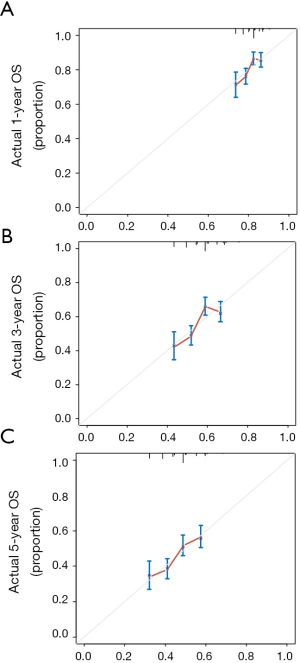
The COX hazards regression analysis showed that age (P<0.001), stage (P=0.003) and treatments (P=0.021) were predictors of OS in univariate analysis (Table 2). All the covariates with P<0.1 in univariate analysis were included into the multivariate analysis, and the results revealed that age (P=0.001), stage (P<0.001) and treatments (P=0.002) were independent factors to predict survival, which strongly suggested that post-operative chemotherapy was recommended for the patients with stage IB or II of LCC. A nomogram including the variables independently related to the survival was shown in Figure 4. The 1-, 3- and 5-year OS could be estimated by adding the points which are corresponding to the patient’s characteristics. The C-index for the nomogram to predict OS was 0.581 (95% CI: 0.557–0.605). Calibration plots of the nomogram prediction accuracy were presented in Figure 5.

Full table
Discussion
Most LCC studies are case reports or have small sample size due to its rarity. Therefore, little is known about the clinical features and prognosis of LCC. In the present research, a total of 1,099 patients with stage I/II LCC were included and retrospectively analyzed.
It has been revealed that LCC mainly occurs in the elderly, and it is more common in males (1,5), which was consistent with our results. Sun et al. (17) had reported that the tumors mostly located in the left lung, which was contrary to our findings. This might be related to the small sample size in their study (n=46). Surprisingly, the primary LCC in most of the patients located in the upper lobe, followed by the lower lobe in our study.
Surgical resection is the first-line treatment for stage I or II NSCLC (5). In view of the unsatisfactory prognosis of LCC, for the early stage LCC, the surgical resection may not definitely achieve favorable prognosis. Gu et al. (18) and Lo Russo et al. (19) found that surgery combined with post-operative chemotherapy had a better prognosis compared to surgery alone for large cell neuroendocrine carcinoma, a subtype of LCC. Similarly, our study showed that the 5-year survival rate of LCC patients with surgery alone was 44.6%, while that was 50.7% in those with post-operative chemotherapy. Furthermore, our study also found no significant difference in the survival rate between patients with or without post-operative chemotherapy among patients with stage IA LCC (P=0.877). However, for patients with LCC at higher stage (IB or higher), the post-operative chemotherapy achieved a better OS than the surgery alone (P=0.006). This was consistent with the results reported by Raman et al. (20).
There are several limitations in this study. This was a retrospective study, and there was limited information on the treatments. Furthermore, the regimens of chemotherapy were unclear. However, this seems to have little effect on our analysis of the effects of postoperative chemotherapy or not.
Up to now, no peculiar or standard chemotherapy regimens for LCC were recommended in the guidelines of NSCLC treatment, and at which exact stage patients with early LCC should receive adjacent chemotherapy after surgery still lacks convincing clinical evidence. To the best of our knowledge, this research was the first and largest retrospective analysis of LCC to determine the efficacy of adjuvant chemotherapy after surgery in early pulmonary LCC. By our analysis, clinicians can better understand the clinicopathological characteristics, survival, and treatment of patients with early pulmonary LCC.
In conclusion, our study indicated that LCC has a higher incidence in the elderly and males, and tends to present poor differentiation. For early stage LCC, surgery combined with chemotherapy should be performed from stage IB, instead of stage II. However, more prospective studies are needed to confirm our findings.
Acknowledgments
Funding: Supported by Medical guidance project of Shanghai Committee on Science and Technology (No. 134119a3400) and Rising Frontiers projects of Shen Kang centre (SHDC12016106).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval was waived by the local ethics committee, as SEER data is publicly available and de-identified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pelosi G, Barbareschi M, Cavazza A, et al. Large cell carcinoma of the lung: a tumor in search of an author. A clinically oriented critical reappraisal. Lung Cancer 2015;87:226-31. [Crossref] [PubMed]
- Driver BR, Portier BP, Mody DR, et al. Next-Generation Sequencing of a Cohort of Pulmonary Large Cell Carcinomas Reclassified by World Health Organization 2015 Criteria. Arch Pathol Lab Med 2016;140:312-7. [Crossref] [PubMed]
- Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005;40:90-7. [Crossref] [PubMed]
- Liu R, Liu J, Shi T, et al. Clinicopathological and genetic characteristics of pulmonary large cell carcinoma under 2015 WHO classification: a pilot study. Oncotarget 2017;8:100754-63. [PubMed]
- Hanagiri T, Oka S, Takenaka S, et al. Results of surgical resection for patients with large cell carcinoma of the lung. Int J Surg 2010;8:391-4. [Crossref] [PubMed]
- Watanabe R, Ito I, Kenmotsu H, et al. Large cell neuroendocrine carcinoma of the lung: is it possible to diagnose from biopsy specimens? Jpn J Clin Oncol 2013;43:294-304. [Crossref] [PubMed]
- Sholl LM. Large-cell carcinoma of the lung: a diagnostic category redefined by immunohistochemistry and genomics. Curr Opin Pulm Med 2014;20:324-31. [Crossref] [PubMed]
- Nagasaka M, Gadgeel S. M. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther 2018;18:63-70. [Crossref] [PubMed]
- Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015.CD011430. [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]
- Park HJ, Park HS, Cha YJ, et al. Efficacy of adjuvant chemotherapy for completely resected stage IB non-small cell lung cancer: a retrospective study. J Thorac Dis 2018;10:2279-87. [Crossref] [PubMed]
- Li X, Zhang C, Sun Z, et al. Propensity-matched analysis of adjuvant chemotherapy for completely resected Stage IB non-small-cell lung cancer patients. Lung Cancer 2019;133:75-82. [Crossref] [PubMed]
- Malhotra J, Mhango G, Gomez JE, et al. Adjuvant chemotherapy for elderly patients with stage I non-small-cell lung cancer >/=4 cm in size: an SEER-Medicare analysis. Ann Oncol 2015;26:768-73. [Crossref] [PubMed]
- Morgensztern D, Du L, Waqar SN, et al. Adjuvant Chemotherapy for Patients with T2N0M0 NSCLC. J Thorac Oncol 2016;11:1729-35. [Crossref] [PubMed]
- Hung JJ, Wu YC, Chou TY, et al. Adjuvant Chemotherapy Improves the Probability of Freedom From Recurrence in Patients With Resected Stage IB Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1346-53. [Crossref] [PubMed]
- Detterbeck FC. The eighth edition TNM stage classification for lung cancer: What does it mean on main street? J Thorac Cardiovasc Surg 2018;155:356-9.
- Sun YH, Lin SW, Hsieh CC, et al. Treatment outcomes of patients with different subtypes of large cell carcinoma of the lung. Ann Thorac Surg 2014;98:1013-9. [Crossref] [PubMed]
- Gu J, Gong D, Wang Y, et al. The demographic and treatment options for patients with large cell neuroendocrine carcinoma of the lung. Cancer Med 2019;8:2979-93. [PubMed]
- Lo Russo G, Pusceddu S, Proto C, et al. Treatment of lung large cell neuroendocrine carcinoma. Tumour Biol 2016;37:7047-57. [Crossref] [PubMed]
- Raman V, Jawitz OK, Yang CJ, et al. Adjuvant Therapy for Patients With Early Large Cell Lung Neuroendocrine Cancer: A National Analysis. Ann Thorac Surg 2019;108:377-83. [Crossref] [PubMed]