Assessment of ovarian reserve by serum anti-Müllerian hormone in patients with systemic lupus erythematosus: a meta-analysis
Introduction
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disorder of unknown pathogenesis with variable course and prognosis. SLE is more often found in adult women, especially of those in reproductive age. However, female SLE patients tend to have a smaller average size of family, which is possibly related to the use of cytotoxic medications as well as psychosocial effects and not related to SLE (1).
Cyclophosphamide (CYC) is the immunosuppressant drug, which is often used in the therapy of SLE. However, some studies have indicated that the treatment of CYC on SLE patients was associated with menstrual disorders or amenorrhea, which is related to the dose and age (2,3). An article written by Ataya et al. (4) demonstrated that CYC affected the rat ovary structure and function and CYC-induced ovarian toxicity was targeted on the granulosa cells, which may explain the ovarian failure caused by CYC. So SLE patients under the therapy of CYC may have a problem on fertility.
Anti-Müllerian hormone (AMH) was originally showed by Jost (5) and is a member of the TGF-β family (6). AMH is expressed in the ovarian granulosa cells, which occurs at the end of fetal life for the first time (7). AMH could be detected at birth and increases steadily in childhood after a transient increase in infancy (8). The serum concentration of AMH reaches the maximum level in puberty and then it gradually decreases throughout reproductive period, reaching undetectable level in menopause (9). Recently, AMH is widely used to evaluate the ovarian reserve since it could be easily measured and is stable through the whole menstrual cycle compared with follicle stimulating hormone (FSH), luteinizing hormone (LH) and estradiol (E2) (10). Furthermore, van Disseldorp et al. showed that AMH demonstrated less individual variation in intra- and inter-cycle than antral follicle count (AFC) (11). Therefore, AMH is a reliable cycle-independent marker for ovarian reserve.
We assume that there might be a relationship between SLE and serum AMH because AMH is a good biomarker of ovarian reserve and SLE patients have adverse maternal and obstetrical outcomes. Recently, some studies have indicated that serum AMH levels were lower in SLE patients than in general population (12-14). On the contrary, Li et al. showed an opposite view (15). Besides, some articles demonstrated that there was no difference on serum AMH levels between SLE patients and general population (16,17). AS for the association between serum AMH levels and the use of CYC, some studies (18,19) referred that serum AMH levels were lower in SLE patients with the treatment of CYC while some indicated that CYC had little influence on serum AMH levels (20,21). Studies with small sample sizes lack statistical power and have resulted in contradictory results. Meta-analysis is a way to increase the effective sample size by collecting data from individual related studies and enhance the statistical power of the analysis. The objective of our study is to perform a systemic review and meta-analysis to confirm the relationship between SLE and ovarian reserve reflected by serum AMH levels as well as the effect of CYC on ovarian reserve of SLE patients.
Methods
Literature and search strategy
PubMed, Embase, Web of Science, CNKI, CHINESE WANFANG, China Science and Technology Database (VIP) databases were searched for eligible studies from the time the databases were established to April 2019 using the combination of the following terms: systemic lupus erythematosus, SLE, anti Müllerian hormone and AMH. All eligible articles were retrieved and their references were reviewed for additional relevant articles. All studies were selected by two independent reviewers and disagreements were solved by discussion.
Inclusion criteria
Studies were included in this meta-analysis if they fulfilled the following inclusion criteria: (I) serum AMH levels were compared between SLE patients and healthy controls or SLE patients with and without the use of CYC; (II) the data of serum AMH levels were available (mean/standard deviation or median/range or median/interquartile interval was provided); (III) were written in English or Chinese. If conference abstracts and published full-length articles were carried out on the same population, the latter were included. Studies without available abstracts or full articles were excluded.
Data extraction and statistical analysis
When the included studies provided serum AMH levels by medians and ranges (or interquartile interval) instead of means and standard deviations, we calculated the means and standard deviations by estimation methods (22). The statistical software R was used during the data estimation.
Standardized mean differences (SMD) with 95% confidence intervals (CIs) was calculated to evaluate the association between serum AMH levels and SLE (P<0.05 was considered statistically significant). Heterogeneity of effects across studies was assessed using the chi-square statistic and quantified by I2. I2 values of 25%, 50% and 70% were considered as low, moderate and high heterogeneity, respectively (23). If I2>50%, the random-effects model was used. Otherwise, the fixed-effects model was used. If statistical heterogeneity existed, the Galbraith plot was applied to detect potential sources of heterogeneity. What’s more, subgroup analyses were carried out according to region, diagnostic criteria for SLE and detection methods of serum AMH levels. Sensitivity analysis was conducted to evaluate the stability of the meta-analysis by sequentially excluding one study at a time. To evaluate the presence of potential publication bias, Begg’s test and funnel plot were performed, and P<0.05 was considered to represent statistically significant publication bias (24). All statistical analyses were performed with STATA 12.0 software.
Results
Characteristics of the included studies
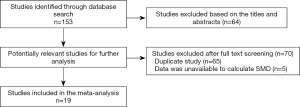
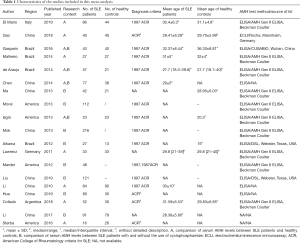
A total of 153 studies were identified at the beginning, and 64 articles were excluded based on the screening of titles or abstracts. Full-text reading was performed for the rest of the 89 potential studies, and details of the searches are shown in the flow chart (Figure 1). Finally, 19 studies that fulfilled all the selection criteria were included in this meta-analysis (12-21,25-33). The characteristics of the included studies are shown in Table 1. Totally, 1,272 SLE patients and 555 healthy controls were included in our study. In the 19 studies, 11 (12-17,25-27,31,33) explored the different serum levels of AMH between SLE patients and healthy controls and 12 (14,17-21,25,26,28-30,32) detected the changes of serum AMH levels between SLE patients with and without the use of CYC. Notably, ng/mL or µg/L was used as unit of AMH in all of the included studies with exception of one written by Hua et al. in which authors used g/L (30). We contacted the first author and he explained that the unit of AMH should be ng/mL in their article, and the misuse is due to their carelessness. Therefore, this article was finally included in this meta-analysis.
Full table
Data analysis
Comparison of serum AMH levels between SLE patients and healthy controls
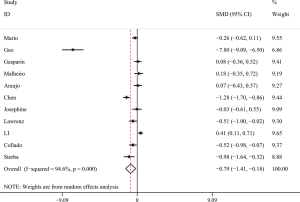
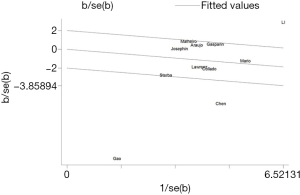
The forest plot for a comparison of serum AMH levels between SLE patients and healthy controls is shown in Figure 2. The I2 was high, so the random-effects model was used. The pooled SMD was −0.79 (95% CI, −1.41 to −0.18) (P<0.05), which showed that the levels of AMH were significantly lower in SLE patients compared with healthy controls. The Galbraith plot was used to find the potential heterogeneity, and three articles (12,15,25) based on Chinese population seemed to be the major source of the heterogeneity (Figure 3). Subgroup analysis found that AMH levels were significantly lower in SLE patients compared with healthy controls in both Chinese and foreign populations. Of the 11 studies, 6 studies (13,14,16,26,27,31) used enzyme-linked immunosorbent assay (ELISA) (AMH Gen II from Beckman Coulter) to measure serum AMH levels, while the rest 5 (12,15,17,25,33) used other methods/kits (shown in Table 1). The pooled SMD of the 6 studies using ELISA was −0.21 (95% CI, −0.40 to −0.02) (P<0.05) with I2 of 28.9%.
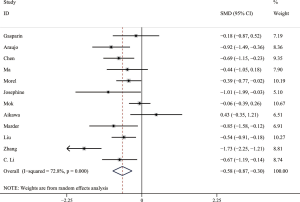
Comparison of serum AMH levels between SLE patients with and without CYC therapy
Owing to significant heterogeneity, we used the random-effects model. The result showed that SLE patients with CYC therapy had significantly lower serum AMH levels than those without CYC therapy. The pooled SMD was −0.58 (95% CI, −0.87 to −0.30) (P<0.05) (shown in Figure 4). The Galbraith plot was used to analyze the source of heterogeneity, and 3 articles (19,21,30) seemed to be the major source of heterogeneity. Of the 12 studies, 6 studies (14,18-20,26,28) used ELISA (AMH Gen II from Beckman Coulter) to measure serum AMH levels and the pooled SMD was −0.51 (95% CI, −0.82 to −0.19) (P<0.05) with I2 of 52.0%. The results remained similar in other subgroups.
Sensitivity analysis
We performed a sensitivity analysis by sequential omission of individual studies. When serum AMH levels were compared between SLE patients and healthy controls, the result became insignificant after the study published by Gao et al. (12) was excluded (P>0.05). The estimate of the pooled SMD was not significantly influenced in a comparison of serum AMH levels between SLE patients with and without the use of CYC.
Publication bias
Funnel plot and Begg’s test were conducted to evaluate the publication bias. No obvious funnel plot asymmetry was found and all the P values of the Begg’s tests were over 0.05, suggesting the publication bias was not evident in our meta-analysis.
Discussion
To our knowledge, this is the first systemic review and meta-analysis to assess the relationship between SLE and ovarian reserve. In this study, we found that SLE is associated with low AMH levels. Furthermore, AMH levels are lower in SLE patients treated with CYC.
Ovarian reserve, predicting fertility potential of women, is represented by the quantity and quality of remaining oocytes (34). To evaluate ovarian reserve, AFC, FSH, AMH and other parameters are often used. Among them, AMH has high sensitivity and specificity in reflecting ovarian response (35). AMH, which is produced by granulosa cells of early follicles, is gonadotropin-independent. Therefore, serum AHM levels remain steady within and between menstrual cycles. In a study involving twelve healthy female subjects aged 18 to 24 years old, the highest and lowest values of serum AMH were 3.9±1.3 and 3.4±1.1 ng/mL, respectively, suggesting stability of serum AMH concentration throughout the menstrual cycle (36). Other studies also showed that AMH levels were relatively consistent during the menstrual cycle (37,38). Furthermore, use of contraceptive has no significant effect on serum AMH levels in healthy women and those with polycystic ovary syndrome (39,40). Overall, AMH is a cost-effective and reliable marker of ovarian reserve.
SLE is a chronic autoimmune disease with various clinical manifestations. It has an obvious female predilection and women in childbearing ages are mainly affected. A case-control study including 94 SLE patients and 40 healthy controls found that menstrual cycle disorders were observed in 54% of SLE patients and were related to SLE disease activity, indicating that SLE women tended to have ovarian dysfunction (41). Our meta-analysis confirmed the conclusion above by comparing serum AMH levels between SLE patients and healthy controls with the pooled SMD of −0.79 (95% CI, −1.41 to −0.18). Actually, SLE could cause systemic inflammation and ovary might be involved, like autoimmune oophoritis, which could lead to the reduction of ovarian function. Chronic inflammation also leads to the dysfunction of the hypothalamic pituitary ovarian (HPO) axis. In addition, SLE itself could cause dysfunction of the HPO axis, resulting in higher serum prolactin and FSH along with lower progesterone and LH levels. The imbalance of hormone could further refer to ovarian dysfunction ending in infertility, menstrual irregularity and ovarian failure (42). However, current studies regarding the relationship between SLE and serum AMH levels are conflicting, which might be associated with the imbalanced sample sizes of these studies. Compared with them, our results are more reliable with the combined analysis of individual studies.
The heterogeneity in a comparison of serum AHM levels between SLE patients and healthy controls was significantly high and three articles (12,15,25) based on Chinese population seemed to be the major source by the Galbraith plot. When doing subgroup analysis, serum AMH levels remained lower in SLE patients compared with healthy controls both in Chinese and foreign populations. There are various techniques in the measurement of serum AMH levels, which may be one cause of heterogeneity. When sub-analysis was performed, we did not find significant heterogeneity in the subgroup in which the same method was used to evaluate serum AMH levels (ELISA, AMH Gen II from Beckman Coulter). These results indicated that the different measurements of AMH may indeed cause the high heterogeneity.
Immunosuppressive agents are often used in moderate and severe lupus nephritis, central nervous system involvement and other diseases. Among these drugs, CYC has the most destructive influence on the ovary (42). Some studies have demonstrated that ovarian failure is common with the treatment of CYC, which is associated with cumulative dose, long period of treatment and greater age at start of treatment (43,44). Our study showed the consistent view that AMH levels in SLE patients were lower with the use of CYC. Phosphoramide mustard and acrolein are two active metabolites of CYC in the body, and the damage to follicle in the ovary is mainly caused by phosphoramide mustard (45). Actually, it leads to ovarian dysfunction by inducing apoptotic death of the oocytes and somatic granulosa cells (46). In a cohort study (47), the percentage of sustained amenorrhea in SLE patients treated with 0.75 mg/body surface of CYC was 17.5%. However, no sustained amenorrhea was found in SLE patients treated with 0.5 mg/body surface of CYC. This study demonstrated that the cumulative dose of CYC is an important risk factor for ovarian failure. What’s more, AMH levels were found to be associated with the dose of CYC in other articles (29,30). However, Mok et al. (19) indicated that there was no relationship between the dose of CYC and AMH levels. Unfortunately, very few studies included in this meta-analysis provided the information of treated dose of CYC in SLE patients, which was not able to do subgroup analysis. The effect of the dose of CYC on ovarian reserve in SLE patients remains controversial and it needs to be solved in the future.
Study limitations
Some limitations of our study should be considered. First, we only used a single parameter to estimate ovarian reserve but it is known that AMH is a non-invasive as well as sensitive marker of ovarian reserve and is superior to FSH, E2, LH and AFH. Second, the sample sizes of the included studies are normally small, which leads to the controversial results of individual studies. And it limits our ability to draw firm conclusions. Third, when comparing serum AMH levels between SLE patients with and without the use of CYC, the heterogeneity is high. Although we did subgroup analyses with the confounders including region, diagnostic criteria of SLE and technique of AMH measurements, the value of I2 remained high. Fourth, when performing sensitivity analysis in a comparison of serum AMH levels between SLE patients and healthy controls, the result was not statistically significant when the study published by Gao et al. (12) was excluded (P>0.05). This might also be due to the small sample sizes of the studies included in the meta-analysis. So, our results are not stable and more studies with large sample sizes should be performed in the future.
Conclusions
Our meta-analysis demonstrates that SLE is related to increased risk of decreased ovarian reserve. Additionally, the treatment of CYC can do harm to ovarian reserve. SLE patients especially women in reproductive ages should undergo serum AMH level measurements to help them make strategies for therapeutic decisions and ovarian preservation.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vinet E, Pineau C, Gordon C, et al. Systemic lupus erythematosus in women: impact on family size. Arthritis Rheum 2008;59:1656-60. [Crossref] [PubMed]
- Gonzalez-Crespo MR, Gomez-Reino JJ, Merino R, et al. Menstrual disorders in girls with systemic lupus erythematosus treated with cyclophosphamide. Br J Rheumatol 1995;34:737-41. [Crossref] [PubMed]
- Boumpas DT, Austin HA 3rd, Vaughan EM, et al. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med 1993;119:366-9. [Crossref] [PubMed]
- Ataya KM, Valeriote FA, Ramahi-Ataya AJ. Effect of cyclophosphamide on the immature rat ovary. Cancer Res 1989;49:1660-4. [PubMed]
- Jost A. Recherches sur la differenciation sexuelle de l’embron de lapin. III Role des gonades foetales dans la differenciation sexuelle somatique. Arch Anat Microsc Morphol Exp 1947;36:271-315.
- Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Mullerian inhibiting substance and expression of the human gene in animal cells. Cell 1986;45:685-98. [Crossref] [PubMed]
- Rajpert-De Meyts E, Jorgensen N, Graem N, et al. Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab 1999;84:3836-44. [PubMed]
- Bhide P, Pundir J, Homburg R, et al. Biomarkers of ovarian reserve in childhood and adolescence: A systematic review. Acta Obstet Gynecol Scand 2019;98:563-72. [Crossref] [PubMed]
- La Marca A, De Leo V, Giulini S, et al. Anti-Mullerian hormone in premenopausal women and after spontaneous or surgically induced menopause. J Soc Gynecol Investig 2005;12:545-8. [Crossref] [PubMed]
- Hehenkamp WJ, Looman CW, Themmen AP, et al. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab 2006;91:4057-63. [Crossref] [PubMed]
- van Disseldorp J, Lambalk CB, Kwee J, et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod 2010;25:221-7. [Crossref] [PubMed]
- Gao H, Ma J, Wang X, et al. Preliminary study on the changes of ovarian reserve, menstruation, and lymphocyte subpopulation in systemic lupus erythematosus (SLE) patients of childbearing age. Lupus 2018;27:445-53. [Crossref] [PubMed]
- Malheiro OB, Rezende CP, Rocha AL, et al. Regular menstrual cycles do not rule out ovarian damage in adult women with systemic lupus erythematosus. Gynecol Endocrinol 2014;30:701-4. [Crossref] [PubMed]
- de Araujo DB, Yamakami LY, Aikawa NE, et al. Ovarian reserve in adult patients with childhood-onset lupus: a possible deleterious effect of methotrexate? Scand J Rheumatol 2014;43:503-11. [Crossref] [PubMed]
- Li Y, Zhao Y, Ci CZ, et al. Anti-Müllerian hormone: its significance in female lupus patients and relation with anti-ovarian antibody. Chinese Journal of Rheumatology 2010;14:305-7.
- Di Mario C, Petricca L, Gigante MR, et al. Anti-Mullerian hormone serum levels in systemic lupus erythematosus patients: Influence of the disease severity and therapy on the ovarian reserve. Endocrine 2019;63:369-75. [Crossref] [PubMed]
- Gasparin AA, Souza L, Siebert M, et al. Assessment of anti-Mullerian hormone levels in premenopausal patients with systemic lupus erythematosus. Lupus 2016;25:227-32. [Crossref] [PubMed]
- Morel N, Bachelot A, Chakhtoura Z, et al. Study of anti-Mullerian hormone and its relation to the subsequent probability of pregnancy in 112 patients with systemic lupus erythematosus, exposed or not to cyclophosphamide. J Clin Endocrinol Metab 2013;98:3785-92. [Crossref] [PubMed]
- Mok CC, Chan PT, To CH. Anti-mullerian hormone and ovarian reserve in systemic lupus erythematosus. Arthritis Rheum 2013;65:206-10. [Crossref] [PubMed]
- Ma W, Zhan Z, Liang X, et al. Subclinical impairment of ovarian reserve in systemic lupus erythematosus patients with normal menstruation not using alkylating therapy. J Womens Health (Larchmt) 2013;22:1023-7. [Crossref] [PubMed]
- Aikawa NE, Sallum AM, Pereira RM, et al. Subclinical impairment of ovarian reserve in juvenile systemic lupus erythematosus after cyclophosphamide therapy. Clin Exp Rheumatol 2012;30:445-9. [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj 2003;327:557-60. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Chen D, Yuan S, Zhan Z, et al. Assessment of ovarian reserve with anti-Mullerian hormone in female patients with systemic lupus erythematosus. Zhonghua Yi Xue Za Zhi 2014;94:977-80. [PubMed]
- Isgro J, Nurudeen SK, Imundo LF, et al. Cyclophosphamide exposure in pediatric systemic lupus erythematosus is associated with reduced serum anti-mullerian hormone levels. J Rheumatol 2013;40:1029-31. [Crossref] [PubMed]
- Lawrenz B, Henes J, Henes M, et al. Impact of systemic lupus erythematosus on ovarian reserve in premenopausal women: evaluation by using anti-Muellerian hormone. Lupus 2011;20:1193-7. [Crossref] [PubMed]
- Marder W, McCune WJ, Wang L, et al. Adjunctive GnRH-a treatment attenuates depletion of ovarian reserve associated with cyclophosphamide therapy in premenopausal SLE patients. Gynecol Endocrinol 2012;28:624-7. [Crossref] [PubMed]
- Liu X, Zhang LL, Zhao W, et al. Effect of cyclophosphamide on ovarian function in patients with systemic lupus erythematosus in childbearing age. Zhonghua Yi Xue Za Zhi 2019;99:174-7. [PubMed]
- Hua Z, Li W, Lipeng L, et al. Evaluation of ovarian reserve function in patients with systemic lupus erythematosus by anti-Mueller hormone. Chinese Journal of Primary Medicine and Pharmacy 2019;26:337-40.
- Collado MV, De Zárate DO, Mayer M, et al. Measurement of antimüllerian hormone as a parameter of ovarian reserve in lupus patients. Relationship with ethnicityand exposure to immunosuppressants. J Clin Rheumatol 2018;24:S26-7.
- Li C, Xie J, Wang X, et al. Strong reduction of anti-müllerian hormone in systemic lupus erythematosus woman of reproductive age. Ann Rheu Dis 2017;76:1226-7.
- Sterba Y, Tanner T, Wahezi D. Evaluation of ovarian reserve and function in adolescent females with systemic lupus erythematosus. Arthritis Rheumatol 2016;68:3233-4.
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril 2015;103:e9-17. [Crossref] [PubMed]
- Broer SL, Dolleman M, Opmeer BC, et al. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update 2011;17:46-54. [Crossref] [PubMed]
- La Marca A, Stabile G, Artenisio AC, et al. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod 2006;21:3103-7. [Crossref] [PubMed]
- Fanchin R, Taieb J, Lozano DH, et al. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod 2005;20:923-7. [Crossref] [PubMed]
- Tsepelidis S, Devreker F, Demeestere I, et al. Stable serum levels of anti-Mullerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod 2007;22:1837-40. [Crossref] [PubMed]
- Streuli I, Fraisse T, Pillet C, et al. Serum antimullerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril 2008;90:395-400. [Crossref] [PubMed]
- Somunkiran A, Yavuz T, Yucel O, et al. Anti-Mullerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2007;134:196-201. [Crossref] [PubMed]
- Shabanova SS, Ananieva LP, Alekberova ZS, et al. Ovarian function and disease activity in patients with systemic lupus erythematosus. Clin Exp Rheumatol 2008;26:436-41. [PubMed]
- Oktem O, Guzel Y, Aksoy S, et al. Ovarian function and reproductive outcomes of female patients with systemic lupus erythematosus and the strategies to preserve their fertility. Obstet Gynecol Surv 2015;70:196-210. [Crossref] [PubMed]
- McDermott EM, Powell RJ. Incidence of ovarian failure in systemic lupus erythematosus after treatment with pulse cyclophosphamide. Ann Rheum Dis 1996;55:224-9. [Crossref] [PubMed]
- Park MC, Park YB, Jung SY, et al. Risk of ovarian failure and pregnancy outcome in patients with lupus nephritis treated with intravenous cyclophosphamide pulse therapy. Lupus 2004;13:569-74. [Crossref] [PubMed]
- Plowchalk DR, Mattison DR. Phosphoramide mustard is responsible for the ovarian toxicity of cyclophosphamide. Toxicol Appl Pharmacol 1991;107:472-81. [Crossref] [PubMed]
- Oktem O, Oktay K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res 2007;67:10159-62. [Crossref] [PubMed]
- Appenzeller S, Blatyta PF, Costallat LT. Ovarian failure in SLE patients using pulse cyclophosphamide: comparison of different regimes. Rheumatol Int 2008;28:567-71. [Crossref] [PubMed]