The efficacy of vitamin D therapy for patients with COPD: a meta-analysis of randomized controlled trials
Introduction
Chronic obstructive pulmonary disease (COPD), one of the top ten leading causes of death worldwide (1), is characterized by progressive and persistent airflow obstruction accompanied by an enhanced chronic inflammatory response and is mainly caused by environmental exposure to smoke and smoking (2). COPD, which is not only treatable, but preventable, represents an important public health challenge. The majority of COPD cases (85%) are smoking-related (3). Exposure to tobacco smoke causes changes in pulmonary function by impeding growth, decreasing peak function and accelerating age-related decline (4). Although lots of drugs, such as theophylline, long-acting beta2-agonists (LABA), inhaled corticosteroids (ICS), can effectively manage COPD, the disease remains the fourth leading cause of mortality in the world (5) and is projected to be the third leading cause of death by 2020. Currently, vitamin D is regarded as a sort of medicine which can play an important role for COPD patients and has certain related systemic effects (6). Furthermore, due to its effects on gene regulation, vitamin D has protective effects against pulmonary diseases (7).
Vitamin D, a form of fat-soluble steroid hormone with biological functions and receptors in various organs, exerts powerful effects on the human body (8,9). These effects are seen particularly in the myometrium and tumor tissue (10). In humans, vitamin D is mostly produced through endogenous cutaneous synthesis of pre-vitamin D3. This synthesis is derived from 7-dehydrocholesterol via exposure to ultraviolet radiation (11). Fatty fish and fish liver oils are the best sources of vitamin D, and beef liver, cheese, and egg yolks also have small amounts of the vitamin (12,13). Vitamin D in these foods is mainly in the form of vitamin D3 (14). Vitamin D is widely known for its functions in calcium and bone metabolism. However, studies in recent decades have suggested that vitamin D has a far broader range of physiological functions and is related to the existence of vitamin D receptors (VDR) on the cells of these tissues, with effects on muscle function and the immune system (15). These functions could have clinical implications for COPD patients. Notably, many COPD patients experience vitamin D deficiency, with the content of vitamin D being closely associated with lung function, inflammatory factors and prognosis (3). Moreover, increased rates of exacerbation and hospitalization in COPD patients are attributed to vitamin D deficiency (16,17). Recent meta-analyses have concluded that vitamin D deficiency is directly related to the severity of a patient’s COPD and, therefore, acute exacerbation may be prevented with vitamin D supplementation (18-20). Some studies have suggested that prognosis for COPD patients who are suffering from respiratory tract infections may improve through correction of their serum vitamin D level (21).
Although the clinical value of vitamin D supplementation in the treatment of COPD has been demonstrated in many studies, several of these had insufficient samples or inconsistent results. To provide accurate evidence for clinical research, we conducted a meta-analysis to investigate whether vitamin D can improve COPD assessment test (CAT) score, sputum, acute exacerbation, 6-minute walk distance (6MWD), or lung function, such as FEV1 and FEV1/FVC, in COPD patients.
Methods
Inclusion criteria
- The study should have been conducted as a randomized controlled trial (RCT);
- Patients must be diagnosed as COPD according to the Guidelines for the Diagnosis and Treatment of COPD;
- he study should explore the relationship between vitamin D and COPD;
- Observational index: FEV1, FEVI/FVC, acute exacerbation, sputum, 6MWD, CAT score.
Exclusion criteria
- The study’s results were affected by a small sample size;
- The study included non-compliant patients or people who had been deficient in vitamin d since childhood;
- The study failed to follow the principles of a randomized-controlled trial (rct);
- References clearly did not meet the eligibility criteria;
- The Jadad score was less than two.
Search methods
We executed a comprehensive search of the electronic databases PubMed, China National Knowledge Internet (CNKI), Embase, Web of Science and Wanfang Data. Searches were conducted using key words including ‘vitamin D’, ‘sputum’, ‘CAT’, ‘lung function, ‘6-minute walking distance’, ‘exacerbation’ and ‘chronic obstructive pulmonary disease’. There were no language restrictions.
Study selection and data extraction
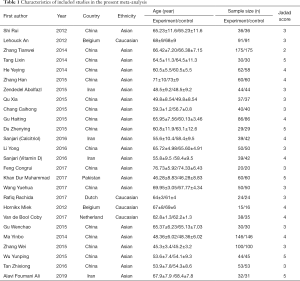
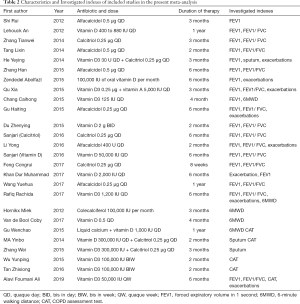
Two independent authors browsed for studies of relevance based on their titles and abstracts. The authors then read the full texts of the potentially relevant studies, applying the inclusion and exclusion criteria. When disagreements arose, a consensus was reached through discussion or re-evaluation by the third author. As shown in Tables 1 and 2, the content for inclusion was: first author, publication year, sample size of experimental and control group, study type, and outcome indicators.
Full table
Full table
Quality appraisal
The Cochrane Collaboration tool was used to assess the included studies. Two independent authors evaluated the quality of each study. Divergences were decided by a senior author. The quality evaluation of the literature considered five biases: selection bias, withdraw bias, performance bias, publication bias, and other bias. Moreover, the methodology quality assessment used by the Jadad score in each study should have been more than two.
Statistical analysis
Meta-analysis was carried out by Review Manager Version 5.3 (Revman 5.3). We used standardized mean difference (SMD) and mean difference (MD) to assess the efficacy of vitamin D therapy in patients with COPD. There was a statistically significant difference when P<0.05. The I2 statistic was used to evaluate heterogeneity of the included studies. In the absence of significant heterogeneity, we used a fixed-effect model (P≥0.05, I2≤50%), otherwise a random-effect model was adopted.
Results
Study characteristics
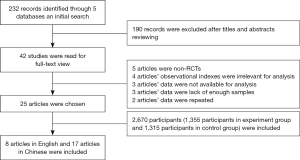
Searches of the PubMed, CNKI, Embase, Web of Science and WanFang Data databases returned 232 potentially relevant records. After their titles or abstracts were read, 190 of these records were excluded. The remaining 42 studies were read and the inclusion and exclusion criteria were applied. From these studies: 5 articles were non-RCTs, 4 articles had observational indexes not related to this meta-analysis, 3 articles did not provide the date required for this meta-analysis, 3 articles were excluded because of insufficient sample sizes, and 2 articles were duplicates. Finally, 26 studies of 25 articles (22-45), with a total of 2,670 participants (of which 1,355 were in the experiment group and 1,315 were in the control group) were included in this meta-analysis. As shown in Figure 1, 8 articles were written in English (22-29) and 17 were written in Chinese (30-46).
Methodological quality of included studies
The 26 included studies were assessed using the Cochrane Collaboration tool. The quality of each study was evaluated by 2 independent reviewers. Differences were resolved by the third reviewer.
Publication bias
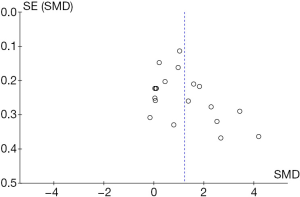
Funnel plots were used to assess the publication bias of the included studies. Both Egger's regression test and Begg’s test were conducted. The Begg’s funnel plot appeared to have a symmetrical shape. No evident publication bias was found in the eligible studies through this analysis (P=0.606 for Egger's regression test, and P=0.315 for Begg’s test) (Figure 2).
Meta-analysis results
FEV1
We collected 19 studies (involving 2,004 patients) from 18 articles which reported the effects of vitamin D supplementation on FEV1 in the experimental group in comparison with the control group (22-26,29-39,42,43). We used a random effects model to assess SMD when the I2 test showed heterogeneity (I2=95%, P<0.01). The results showed a statistically significant difference between the FEV1 of the patients treated with vitamin D supplementation and those treated without. (SMD: 1.21, 95% CI: 0.76–1.66, P<0.01) (Figure 3).
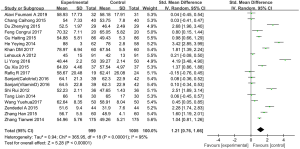
FEV1/FVC
Thirteen studies (involving 1,524 patients from 12 studies] compared FEV1/FVC in the experimental group after vitamin D supplementation with that of the control group (22,25,26,29-36,38,39). Overall, the results demonstrated that vitamin D in patients with COPD had a statistical significance in FEV1/FVC (SMD: 1.07, 95% CI: 0.56–1.58, P<0.01), according to a random effects model (I2=95%, P<0.01) (Figure 4).
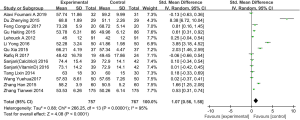
Acute exacerbation
Six articles (involving 539 patients: 272 in the experimental group, and 267 in the control group) showed acute exacerbation in the experimental group after vitamin D supplementation, in comparison with the control group (22-24,30-31,42). Overall, the frequency of acute exacerbation in the experimental group was less than in the control group (SMD: 0.39, 95% CI: 0.23–0.64, P<0.01). As the results showed no heterogeneity, we chose a fixed effects model (I2=0%, P=0.68) (Figure 5).
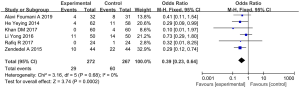
Sputum
In total, 3 articles (involving 612 patients: 308 in the experimental group, and 304 in the control group) showed the effects of vitamin D supplementation on sputum (41-43). The results revealed that sputum in COPD patients with vitamin D supplementation decreased significantly when compared to the control group (SMD: −6.02, 95% CI: −8.25 to −3.79, P<0.01). We used a random effects model to integrate the data (I2=97%, P<0.01) (Figure 6).
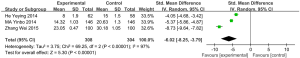
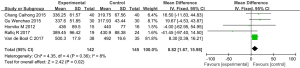
6MWD
Five articles (involving 287 patients: 142 in the experimental group, and 145 in the control group) showed the effects of vitamin D supplementation on the 6-minute walk distance in the experimental group in comparison with the control group (22,27,28,36,40). Due to the absence of significant heterogeneity, we used a fixed effects model (I2=8%, P=0.36). The results indicated a statistically significant difference between the 6MWD of patients treated with vitamin D supplementation and those treated without (MD: 8.82, 95% CI: 1.67–15.98, P=0.02) (Figure 7).
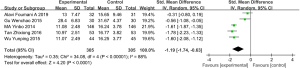
CAT score
Five studies (involving 610 patients: 305 in the experimental group and 305 in the control group), included the COPD assessment test (CAT) score (30,40,41,44,45). Overall, the results demonstrated that there is a statistically significant difference in CAT between those COPD patients treated with vitamin D supplementation and those treated without (SMD: −1.19, 95% CI: −1.74 to −0.63, P<0.01). Because of the significant heterogeneity (I2=88%, P<0.01, we used a random effects model to examine the SMD (Figure 8).
Discussion
Vitamin D, one of the fat-soluble vitamins, is found in two compounds: ergocalciferol (D2) and cholecalciferol (D3). Vitamin D plays a crucial role in bone and mineral metabolism (46) as, by functioning as a negative regulator of the renin–angiotensin–aldosterone system (RAAS), it modulates myocardial extracellular matrix turnover. Thus, vitamin D deficiency can cause the deterioration of heart function and accelerate myocardial remodeling (47). Vitamin D insufficiency is a factor in other conditions, sometimes leading to rickets, osteoporosis and osteomalacia, as well as other bone-related disorders (46). Recently, an increasing number of studies have suggested that vitamin D has a far broader range of physiological functions, which might have clinical implications for COPD patients (22).
Recently, there has been a growing desire to investigate the role of vitamin D in the treatment of respiratory diseases, including asthma and chronic obstructive pulmonary disease (COPD). Some hopeful reports on the possible application of vitamin D as a treatment for asthma have been published (48). Vitamin D, which plays an essential function in the pathogenesis of asthma, reduces the production of inflammatory cytokines via T-helper type-9 lymphocytes, such as interleukin-5 (IL-5), IL-9, and IL-13 (49). Further links between vitamin D and COPD have also been highlighted. Some experimental studies have suggested that the role of vitamin D in the growth and development of COPD can not be ignored. The existence of vitamin D in alveolar type II cells can enhance surfactant synthesis and regulate epithelial-mesenchymal interactions (49). Furthermore, there is a molecular relationship between smoking and vitamin D signaling. Cigarette smoke extracts inhibit VDR translocation in human alveolar epithelial cells, which leads to downregulation of local vitamin D signaling, resulting in deficiency in the control of proinflammatory processes in the airways of COPD patients (50).
In 2017, Rafiq et al. reported that there were no differences in 6-minute walk test results, handgrip strength, pulmonary function, exacerbation rate, or quality of life between COPD patients in the vitamin D group and the placebo group. Taking vitamin D increases the serum 25(OH)D levels (22). Unfortunately, one study alone may have insufficient power to support or suppose the use of vitamin D in COPD patients. Some contributing factors are as follows: (I) the article was a pilot trial, and the major limitation of this study was the small sample size, which only included 50 participants; (II) the study excluded participants aged >70 years, which could have led to a lower mean age of the study population; (III) the study also excluded patients with severe vitamin D deficiency (15 nmol/L), which might have affected the outcomes, as a potential effect of vitamin D supplementation was expected to be stronger in vitamin D-deficient COPD patients. In 2017, Khan et al. also revealed the relationship between the vitamin D and COPD patients (24). In this study, the results demonstrated that prolonged vitamin D use in patients with COPD could reduce the frequency of acute exacerbation and improve FEV1, FVC; however, it couldn’t alleviate dyspnea, sputum volume, or sputum purulence. Khan et al. showed that the dose of vitamin D used to treat COPD patients and the baseline serum vitamin D levels in patients might lead to contradictory results. Furthermore, more studies regarding vitamin D therapy for COPD patients have been emerging (36,41,42). However, their results have been inconclusive due to factors including sample size, participation, the dose of vitamin D, and course of treatment. Therefore, we enforced a comprehensive meta-analysis to evaluate the effects of vitamin D supplementation in patients with COPD.
In our meta-analysis, we found that vitamin D used in patients with COPD can improve lung function (FEV1, FEV1/FVC), 6MWD and reduce the acute exacerbation, sputum, and CAT score. The pathogenesis of COPD mainly includes inflammation, oxidative stress, and pulmonary protease-antiprotease imbalance. A number of articles have reported the relationship between vitamin D and the pathogenesis of COPD. Some previous experimental studies have suggested that the existence of vitamin D in alveolar type II cells promotes surfactant synthesis and regulates epithelial-mesenchymal interactions. Our report (51,52) proved that vitamin D could decrease oxidative stress and particulate matter-induced IL-6 response, and that vitamin D sufficiency was probably beneficial in protecting against pollution-associated diseases related to the induction of airway and systemic inflammation. Smoking was the most important element which led to airway inflammation. The study demonstrated the relationship between smoking and vitamin D signaling (50). Cigarette smoke extracts inhibit VDR translocation in human alveolar epithelial cells and result in downregulation of local vitamin D signaling, which causes deficiency in controlling proinflammatory processes in the airways of COPD patients. This means that by supplying vitamin D to COPD patients, their symptoms could be improved.
At present, theophylline, long-acting beta2-agonists (LABA), inhaled corticosteroids (ICS) are the most commonly used drugs to treat patients with COPD. The main function mechanisms of these drugs is to eliminate inflammation and to relax the bronchial tube to decrease airway resistance. Interestingly, vitamin D sufficiency is perhaps beneficial in protecting against airway and systemic inflammation diseases. Many studies have considered that vitamin D supplementation can improve COPD symptoms. According to previous studies and our meta-analysis, we also could put forward a hypothesis that vitamin D could increase the serum concentration of the previously mentioned drugs and improve patients’ lung function. In this meta-analysis, the included 25 articles did not respectively observe all indexes which we discussed in our study. Different articles might draw different conclusions due to the dose of vitamin D used, the method of administering vitamin D and individual differences, including age, gender and race. However, by analyzing the 25 articles, we can arrive at a general conclusion that, when used in patients with COPD, vitamin D can improve some indexes. The follow points may explain the validity of this conclusion: (I) the articles we chose came from different countries and contained a mass of samples; (II) for differences in the methods of application, dosage and courses of treatment of vitamin D, we carried out a unified and detailed analysis; (III) the 25 articles were chosen in accordance with strict inclusion criteria and exclusion criteria.
However, there were still some limitations of the present meta-analysis when explaining aggregated results. Firstly, each of the included individuals had a different environment and their jobs were not classified, and we lacked sufficient data to perform subgroup analysis to reduce the influence of individual differences. Secondly, several inevitable problems existed within the included studies, such as sample size, age and gender of research objects, and differences in inclusion and exclusion criteria which could cause bias. Thirdly, the articles were published at different periods in time and may be limited by current medical conditions, which could also have affected our results. In this meta-analysis, no subgroup analysis was conducted to factor in the range in vitamin D dosage. Furthermore, the degree of COPD and the baseline level of 25(OH)D in serum each had an impact on the efficiency of vitamin D used in COPD patients and were not addressed. Despite these limitations, we were able to select more articles according to our inclusion criteria to increase sample size to control bias and could confirm results through well designed studies with large sample sizes. We were also able to minimize bias throughout the entire process by creating a detailed protocol, selecting articles independently, and using statistical analysis and data selection. We, therefore, have full confidence in the reliability of our results.
Conclusions
Overall, the results of this meta-analysis showed that treatment with vitamin D may improve the lung function of COPD patients (FEV1, FEV1/FVC), 6MWD and reduce the frequency of acute exacerbation, sputum, and CAT score.
Acknowledgments
Funding: This study was supported by a grant (CYFY2018GLPHX04) from the Special Scientific Research Foundation of the First Affiliated Hospital of Chengdu Medical College in 2018.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was reviewed by the experts of the hospital ethics committee and was approved as complying with the requirements and guidelines of research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151-210. [Crossref] [PubMed]
- Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease website. Available online: Accessed 5th July 2017.http://www.goldcopd.org/guidelines-global-strategy-for-diagnosismanagement.html
- Forey BA, Thornton AJ, Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med 2011;11:36. [Crossref] [PubMed]
- Guerra S, Stern DA, Zhou M, et al. Combined effects of parental and active smoking on early lung function deficits: a prospective study from birth to age 26 years. Thorax 2013;68:1021-8. [Crossref] [PubMed]
- Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol 2009;158:20-5. [Crossref] [PubMed]
- Janssens W, Lehouck A, Carremans C, et al. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med 2009;179:630-6. [Crossref] [PubMed]
- Fletcher JM, Basdeo SA, Allen AC, et al. Therapeutic use of vitamin D and its analogues in autoimmunity. Recent Pat Inflamm Allergy Drug Discov 2012;6:22-34. [Crossref] [PubMed]
- Holick MF, Vitamin D. A millenium perspective. J Cell Biochem 2003;88:296-307. [Crossref] [PubMed]
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. [Crossref] [PubMed]
- Bläuer M, Rovio PH, Ylikomi T, et al. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril 2009;91:1919-25. [Crossref] [PubMed]
- Holick MF. Vitamin D: a d-lightful solution for health. J Investig Med. 2011;59:872-80. [Crossref] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011.
- U.S. Department of Agriculture, Agricultural Research Service (ARS). USDA National Nutrient Database for Standard Reference.
- Ovesen L, Brot C, Jakobsen J. Food contents and biological activity of 25-hydroxyvitamin D: a vitamin D metabolite to be reckoned with? Ann Nutr Metab 2003;47:107-13. [Crossref] [PubMed]
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080S-1086S. [Crossref] [PubMed]
- Malinovschi A, Masoero M, Bellocchia M. Severe vitamin D deficiency is associated with frequent exacerbations and hospitalization in COPD patients. Respir Res 2014;15:131. [Crossref] [PubMed]
- Kokturk N, Baha A, Oh YM, et al. Vitamin D deficiency: what does it mean for chronic obstructive pulmonary disease (COPD)? A comprehensive review for pulmonologists. Clin Respir J 2018;12:382-97. [Crossref] [PubMed]
- Zhu B, Zhu B, Xiao C, et al. Vitamin D deficiency is associated with the severity of COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2015;10:1907-16. [PubMed]
- Zhu M, Wang T, Wang C, et al. The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2016;11:2597-607. [Crossref] [PubMed]
- Martineau AR, James WY, Hooper RL. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med 2015;3:120-30. [Crossref] [PubMed]
- Ginde AA, Mansbach JM, Camargo CA. Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep 2009;9:81-7. [Crossref] [PubMed]
- Rafiq R, Prins HJ, Boersma WG, et al. Effects of daily vitamin D supplementation on respiratory muscle strength and physical performance in vitamin D-deficient COPD patients: a pilot trial. Int J Chron Obstruct Pulmon Dis 2017;12:2583-92. [Crossref] [PubMed]
- Zendedel A, Gholami M, Anbari K, et al. Effects of Vitamin D Intake on FEV1 and COPD Exacerbation: A Randomized Clinical Trial Study. Glob J Health Sci 2015;7:243-8. [Crossref] [PubMed]
- Khan DM, Ullah A, Randhawa FA, et al. Role of Vitamin D in reducing number of acute exacerbations in Chronic Obstructive Pulmonary Disease (COPD) patients. Pak J Med Sci 2017;33:610-4. [Crossref] [PubMed]
- Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2012;156:105-14. [Crossref] [PubMed]
- Sanjari M, Soltani A, Habibi Khorasani A, et al. The effect of vitamin D on COPD exacerbation: a double blind randomized placebo-controlled parallel clinical trial. J Diabetes Metab Disord 2016;15:33. [Crossref] [PubMed]
- Hornikx M, Van Remoortel H, Lehouck A, et al. Vitamin D supplementation during rehabilitation in COPD: a secondary analysis of a randomized trial. Respir Res 2012;13:84. [Crossref] [PubMed]
- van de Bool C, Rutten EPA, van Helvoort A, et al. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J Cachexia Sarcopenia Muscle 2017;8:748-58. [Crossref] [PubMed]
- Alavi Foumani A, Mehrdad M, Jafarinezhad A, et al. Impact of vitamin D on spirometry findings and quality of life in patients with chronic obstructive pulmonary disease: a randomized, double-blinded, placebo-controlled clinical trial. Int J Chron Obstruct Pulmon Dis 2019;14:1495-501. [Crossref] [PubMed]
- Zhang TW, Fu HW, Mao LQ, et al. Effects of vitamin D supplementation on bone mineral density and inflammatory cytokines in COPD patients. 2014;12:37-40.
- Gu HT, Shao HY, Jing XH. Clinical study of alfacalcidol soft capsule on immune function in patients with chronic obstructive pulmonary disease. Chinese Journal of Clinical Pharmacology 2015;31:1373-5.
- Li Y, Chen ZL. Effects of vitamin D on acute exacerbation and mortality in patients with COPD. Modern Practical Medicine 2016;28:314-5.
- Wang YH. Effects of vitamin D on T lymphocyte subsets and lung function in patients with stable COPD. Zhejiang Medical Education 2017;16:52-4.
- Du ZY. Efficacy of vitamin D in the treatment of patients with acute exacerbation of chronic obstructive pulmonary disease complicated by hypocalcemia. Modern Diagnosis and Treatment 2015;26:1763-4.
- Qu X, Han DS, Li YP. The adjuvant therapeutic effect of vitamin A and vitamin D on stable COPD patients. Journal of Practical Medicine 2015;32:995-6.
- Zhang H, Gong JH, Zhang JH, et al. The value of vitamin D in the treatment of stable patients with chronic obstructive pulmonary disease. Laboratory Medicine and Clinical 2015;12:1304-5.
- Chang CH. Effects of vitamin D on chronic obstructive pulmonary disease. Chinese Community Physician 2015;31:15-7.
- Shi R, Huang H, Fang ZY. Effects of vitamin D supplementation on serum 25(OH)D and FEV1 in patients with chronic obstructive pulmonary disease. Journal of Clinical and Experimental Medicine 2012;11:1849-52.
- Feng CR, He LM, Xu G, et al. Effect of vitamin D supplementation on COPD in elderly patients and its effect on serum il-33 expression in patients. Journal of Practical Medicine 2017;34:609-12.
- Tang LX, Zhang Y, Yuan QY. The application of vitamin D in the treatment of patients with chronic obstructive pulmonary disease. International Journal of Laboratory Medicine 2014;35:2317-8.
- Gu WC, Qi GS, Yuan YP. Clinical study on the efficacy of vitamin D in delaying the progression of chronic obstructive pulmonary disease. Chinese Medical Journal 2015;17:1124-5.
- Ma YB. The adjunctive role of vitamin D in the treatment of COPD. Health for all (academic edition) 2014;8:199.
- He YY, Gen TD. The application value of vitamin D in the adjuvant therapy of chronic obstructive pulmonary disease. Journal of Practical Cardiovascular and Cerebrovascular Diseases 2014;22:24-6.
- Zhang W. The adjunctive role of vitamin D in the treatment of COPD. Health Today 2015;14:121.
- Wu YP, Hu QM, Liu W, et al. Effects of vitamin D adjuvant therapy on quality of life in patients with chronic obstructive pulmonary disease. Clinical Meta-analysis 2013;28:569-70.
- Tan ZX, Ye ZT, Fu WQ. Effect of vitamin D on immunomodulatory function and quality of life in patients with copd. Journal of Clinical Pulmonology 2016;21:1062-6.
- Jahanjoo F, Farshbaf-Khalili A, Shakouri SK, et al. Maternal and Neonatal Metabolic Outcomes of Vitamin D Supplementation in Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Ann Nutr Metab 2018;73:145-59. [Crossref] [PubMed]
- Zhao JD, Jia JJ, Dong PS, et al. Effect of vitamin D on ventricular remodelling in heart failure: a meta-analysis of randomized controlled trials. BMJ Open 2018;8:e020545. [Crossref] [PubMed]
- Jiao J, King TS, McKenzie M, et al. Vitamin D3 therapy in patients with asthma complicated by sinonasal disease: Secondary analysis of the Vitamin D Add-on Therapy Enhances Corticosteroid Responsiveness in Asthma trial. J Allergy Clin Immunol 2016;138:589-92.e2. [Crossref] [PubMed]
- Białek-Gosk K, Rubinsztajn R, Białek S, et al. Serum Vitamin D Concentration and Markers of Bone Metabolism in Perimenopausal and Postmenopausal Women with Asthma and COPD. Adv Exp Med Biol 2018;1070:27-36. [Crossref] [PubMed]
- Janssens W, Decramer M, Mathieu C, et al. Vitamin D and chronic obstructive pulmonary disease: hype or reality? Lancet Respir Med 2013;1:804-12. [Crossref] [PubMed]
- Pfeffer PE, Lu H, Mann EH, et al. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS One 2018;13:e0200040. [Crossref] [PubMed]