
Radiofrequency ablation of synchronous multiple primary lung cancer assisted by a magnetic navigation system: a case report
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. The incidence of multiple primary lung cancer (MPLC) has increased in recent years. Such an increase can be explained, in part, by the development of low-dose chest computed tomography (LDCT) screening for lung cancer (1,2). Surgical resection is the standard treatment for early-stage MPLC (3); however, due to multiple nodules located in separate lung lobes and limited pulmonary reserve, a significant proportion of patients with MPLC are ineligible for surgical resection. For those patients who cannot tolerate surgery, local therapy is an optional strategy. Goldberg described successful radiofrequency ablation (RFA) in a lung animal model (4). Dupuy successfully applied RFA in three patients with lung malignancies (5). Recently, 54 medically-inoperable patients with stage IA non-small cell lung cancer (NSCLC) were enrolled from 16 US centers, and those patients achieved an overall survival rate of 86.3% at 1 year and 69.8% at 2 years using CT-guided RFA therapy (6). Nevertheless, CT-guided percutaneous RFA treatment of lung tumors has not been widely accepted in clinical practice because of the high incidence of complications following repeated aspiration and needle adjustments (7). Electromagnetic navigation-guided transthoracic wall positioning system is a new technique for the treatment of multiple primary pulmonary nodules, which can provide accurate pre-operative positioning and real-time intraoperative navigation, avoid important blood vessels and reduce complications.
In the present report, this is first case report of electromagnetic navigation-guided percutaneous RFA for early peripheral lung cancer in a patient with MPLC, which demonstrate the feasibility of the new technique assisting percutaneous RFA for the treatment of early peripheral lung cancer. We present the following case in accordance with the CARE Guideline.
Case presentation
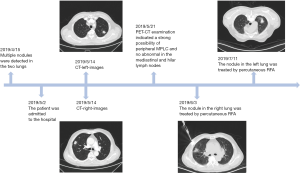
A 68-year-old Chinese man was admitted to the Department of Pulmonary at Shanghai Chest Hospital on 02 May 2019 after multiple nodules were detected in the two lungs. The patient had a 20-year smoking history and a history of a rib fracture in June 2003. The patient was diagnosed with chronic obstructive pulmonary disease in October 2013. Pulmonary function testing demonstrated that the FEV1-to-FVC ratio (FEV1/FVC) was <65.3% of normal; FEV1 was 56.7% of the predicted value. The chest CT examination showed two nodules in both lungs, and multiple malignant neoplasms were not excluded. The nodule in the upper lobe of the right lung was larger (18.74 mm × 11.75 mm) than the nodule in the upper lobe of the left lung (15.41 mm × 10.66 mm). Whole-body positron emission tomography (PET)-CT examination further showed an increase in F18 fluorodeoxyglucose (FDG) metabolism in both of the pulmonary nodules, indicating a strong possibility of peripheral MPLC. No abnormal increase in F18 FDG metabolism was noted in the mediastinal and hilar lymph nodes. Based on the multidisciplinary team discussion, the patient underwent RFA therapy for the targets in the lung, and both of the nodules were confirmed to be malignant based on intra-procedural cytodiagnosis.
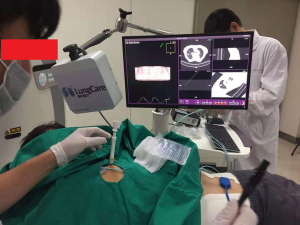
With the guidance of an electromagnetic navigation system (Suzhou LungCare Medical Technologies Inc., China), the nodule in the right lung (Figure 1) and the nodule in the left lung (Figure 1) were treated by percutaneous RFA on 03 Jun 2019 and 11 Jul 2019, respectively. Before RFA therapy, local anesthesia was achieved with intradermal and subcutaneous lidocaine (1%). The sterile electromagnetic position sensor on the needle was locked to search for the entrance point and the right angle to the target was selected based on observation of the transverse and coronal views seen on the electromagnetic system screens. The electromagnetic system generates images in an axial, sagittal, coronal, or oblique plane to assist with needle-probe placement. When the electromagnetic system suggested the tip of the needle was within the nodule, a CT scan was performed for configuration, then fine-needle aspiration cytology of the pulmonary nodules was performed. Two 180-cm2 grounding pads were then placed on the patient’s thighs. Before treatment, the electromagnetic position sensor on the RFA electrode was locked for tracking the tip of the electrode. The areas treated with RFA covered the size of the tumor mass. The patient was treated with a 17-gauge, 3-cm active-tip RFA electrode (cool tip) and generator (Medsphere International Co., Ltd., China) for 10 min per lesion (Figure 2). RFA was initiated at a power of 5W and increased 5W every minute until the temperature reached 95 °C. A post-procedural CT scan was obtained to assess for complications. No post-procedural pneumothorax, hemorrhage, pleural effusion, infection and other complications developed after the procedure.
Discussion
The patient presented in the current report had two nodules in different lung lobes, and was not a candidate for surgical resection due to inadequate pulmonary function. In this case we present the feasibility of percutaneous RFA utilizing a electromagnetic navigation platform.
Local therapy has been shown to be a promising alternative therapy strategy for early-stage lung cancer patients who are poor surgical candidates. Several studies have reported comparable outcomes after stereotactic body radiotherapy SBRT treatment and surgical resections for patients with early stage NSCLC (8-10). SBRT is limited by respiratory movements, and the complication of radiation pneumonitis. Zemlyak et al. (11) demonstrated comparable survival after sub-lobar resection and ablative therapy at 3 years, and suggested that RFA is a promising alternative in high-risk stage I NSCLC patients eligible for surgical resection. The advantage of RFA lies in the ability to locally heat tumors to a lethal temperature while incurring minimal damage to surrounding normal lung tissue (12). Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial demonstrated that RFA is a single, minimally invasive procedure that is well-tolerated in medically-inoperable patients, does not adversely affect pulmonary function testing, and provides a 2-year overall survival rate that is comparable to the rate reported after SBRT in similar patients (6). Indeed, RFA has been widely used via a percutaneous method with widespread application in the treatment of liver, kidney, lung, and bone tumors (13). CT-guided percutaneous RFA has limitations in lung tumor therapy because of the high incidence of complications, such as pneumothoraces, hemothoraces, and bronchopleural fistulas (7). Moreover, this technique cannot be guided in real time, thus it is difficult to accurately locate some small lesions, especially for early peripheral lung tumors <3 cm in size. In most cases, it is necessary to repeatedly scan and adjust the position of the puncture needle, which results in additional radiation exposure for the patient and increased complications.
RFA through bronchoscopy for the treatment of peripheral lung cancer is currently under active exploration. Xie, in our institution, reported that navigation bronchoscopy-guided (ENB) RFA is a safe and feasible procedure for stage IA lung cancer or lung metastasis (14). ENB is a technique in which the bronchoscope is guided through the bronchial tree to accurately reach a peripheral lesion. It has been reported that the diagnostic yield of bronchoscopy can be improved up to 88% by utilizing ENB (15). The NAVIGATE study demonstrated a sensitivity of 69% for malignancy (16). Although the bronchoscopic yield for solitary pulmonary nodules has been dramatically improved with the guide of an electromagnetic navigation system, some peripheral lung cancer is not reachable.
Most early peripheral lung cancers close to the chest wall can be reached by CT-guided transthoracic needle aspiration. Previously, we developed an electromagnetic navigation-guided transthoracic wall positioning system that promises accurate navigation to peripheral pulmonary target lesions. This system provides pre-operative accurate positioning and intra-operative real-time navigation. The electromagnetic navigation-guided transthoracic wall positioning system can shorten the puncture time, avoid important blood vessels, reduce the number of needle adjustments and puncture time, reduce the X-ray radiation dose to the patient, and reduce complications. Several studies have confirmed that electromagnetic navigation is a highly safe, and effective support tool in percutaneous CT-guided lung biopsy (17-19). We have launched a study with a focus on comparing electromagnetic navigation- and CT-guided TTNA in the diagnosis of pulmonary peripheral nodules (NCT03802266).
Electromagnetic navigation-guided percutaneous needle-based RFA has been successfully applied in the treatment of hepatocellular carcinoma (20). In the current case, we utilized the electromagnetic navigation system to assist the clinician in inserting the RFA electrode into lung tumors. During the procedure, there is no needle adjustment or complications, such as pneumothoraces and hemoptysis. We showed the feasibility of applying this technique in the treatment of early-stage lung cancer patients who are ineligible for surgical resection, such as MPLC. The limitation of this report is that we cannot pre-operatively confirm whether or not both of the nodules were MPLC based on histodiagnosis because of the high risk associated with pneumothorax biopsy in emphysema patients. Both of the nodules were confirmed to be malignant based on fine needle aspiration cytology during RFA therapy.
Conclusions
In conclusion, the transthoracic electromagnetic navigation positioning system can be applied in percutaneous RFA for early peripheral lung cancer. Further study is warranted to confirm the efficacy of this technique in the treatment of lung cancer.
Acknowledgments
We would like to thank the patient and his family for the consent of the publication.
Funding: This work was supported by National Key Research and Development Program of the Ministry of Science and Technology of China [2017YFC0112700].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mascalchi M, Comin CE, Bertelli E., et al. Screen-detected multiple primary lung cancers in the ITALUNG trial. J Thorac Dis 2018;10:1058-66. [Crossref] [PubMed]
- Shen C, Che G. A Different Method in Diagnosis of Multiple Primary Lung Cancer. J Thorac Oncol 2016;11:e53-4. [Crossref] [PubMed]
- Yang H, Sun Y, Yao F., et al. Surgical Therapy for Bilateral Multiple Primary Lung Cancer. Ann Thorac Surg 2016;101:1145-52. [Crossref] [PubMed]
- Goldberg SN, Gazelle GS, Compton CC, et al. Radiofrequency tissue ablation in the rabbit lung: efficacy and complications. Acad Radiol 1995;2:776-84. [Crossref] [PubMed]
- Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 2000;174:57-9. [Crossref] [PubMed]
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015;121:3491-8. [Crossref] [PubMed]
- Welborn SL, Ohori NP, Nason KS, et al. Percutaneous computed tomography-guided biopsy performed by thoracic surgeons in 955 patients: A paradigm shift in image-guided thoracic procedures. J Thorac Cardiovasc Surg 2018. pii: S0022-5223(18)32939-8.
- Sebastian NT. Xu-Welliver, MWilliams TM. Stereotactic body radiation therapy (SBRT) for early stage non-small cell lung cancer (NSCLC): contemporary insights and advances. J Thorac Dis 2018;10:S2451-64. [Crossref] [PubMed]
- Rusthoven CG, Jones BL, Kavanagh BD. Medical operability and inoperability drive survival in retrospective analyses comparing surgery and SBRT for early-stage lung cancer. J Thorac Cardiovasc Surg 2018;155:810-1. [Crossref] [PubMed]
- Donovan EK, Swaminath A. Stereotactic body radiation therapy (SBRT) in the management of non-small-cell lung cancer: Clinical impact and patient perspectives. Lung Cancer (Auckl) 2018;9:13-23. [Crossref] [PubMed]
- Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg 2010;211:68-72. [Crossref] [PubMed]
- Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology 2011;260:633-55. [Crossref] [PubMed]
- Ahmed M, Brace CL, Lee FT Jr, et al. Principles of and advances in percutaneous ablation. Radiology 2011;258:351-69. [Crossref] [PubMed]
- Xie F, Zheng X, Xiao B, et al. Navigation Bronchoscopy-Guided Radiofrequency Ablation for Nonsurgical Peripheral Pulmonary Tumors. Respiration 2017;94:293-8. [Crossref] [PubMed]
- Taton O, Bondue B, Gevenois PA, et al. Diagnostic Yield of Combined Pulmonary Cryobiopsies and Electromagnetic Navigation in Small Pulmonary Nodules. Pulm Med 2018;2018:6032974. [Crossref] [PubMed]
- Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J Thorac Oncol 2019;14:445-58. [Crossref] [PubMed]
- Long J, Petrov R, Haithcock B, et al. Electromagnetic Transthoracic Nodule Localization for Minimally Invasive Pulmonary Resection. Ann Thorac Surg 2019;108:1528-34. [Crossref] [PubMed]
- Grand DJ, Atalay MA, Cronan JJ, et al. CT-guided percutaneous lung biopsy: comparison of conventional CT fluoroscopy to CT fluoroscopy with electromagnetic navigation system in 60 consecutive patients. Eur J Radiol 2011;79:e133-6. [Crossref] [PubMed]
- Arias S, Lee H, Semaan R, et al. Use of Electromagnetic Navigational Transthoracic Needle Aspiration (E-TTNA) for Sampling of Lung Nodules. J Vis Exp 2015.e52723. [PubMed]
- Tomonari A, Tsuji K, Yamazaki H, et al. Feasibility of the virtual needle tracking system for percutaneous radiofrequency ablation of hepatocellular carcinoma. Hepatol Res 2013;43:1352-5. [Crossref] [PubMed]


