Pathogen identification in 84 Patients with post-traumatic osteomyelitis after limb fractures
Introduction
Chronic osteomyelitis often results from untreated acute osteomyelitis in children but frequently manifests as post-traumatic osteomyelitis (PTO) in adults (1). It is a serious complication of orthopedic trauma. Use of antibiotics in the acute phase of trauma is essential for preventing postoperative infections (2,3). Chronic osteomyelitis can be caused by persisting bacterial infection after incomplete debridement and/or irrational use of antibiotics (4-6). Treatment of PTO is mainly based on the removal of dead bones and damaged soft tissues, elimination of dead spaces, and the use antibiotics based on bacterial culture findings. However, debridement alone is ineffective in eliminating bacteria colonization in the residual microtubule system of compact bones (7). Bacterial biofilm formation and the generation of antibiotic-resistant bacterial strains can often be accompanied by impaired blood supply at the site of the injury and by inadequate individualized treatment options such as internal plants, bone defects, and wound closure, which make the condition become more prolonged, recurrent, and refractory (2,8-10) .
The passive immunotherapy of targeting the protein components of bacteria has recently garnered intense research focus (11); however, the crucial ability to identify the bacterial trains in different areas at different time points remains elusive due to the diversities in the pathogen hosts and regional distribution (12). Few multicenter randomized controlled trials have investigated the epidemiological data of pathogens in different areas, and there is also a lack of timely and dynamic data that can inform clinical treatment (3). Our hospital is located at the borders of the 3 western provinces of Guizhou, Sichuan, and Chongqing. With limited medical resources and data concerning the causative pathogens of chronic osteomyelitis in its surrounding areas, our hospital faces difficulty in rationally using prophylactic antibiotics. In this article, we summarize the data of 5,268 patients with traumatic fractures of the limbs that were treated in our department from January 1, 2012, to December 31, 2015. According to the inclusion and exclusion criteria, the causative pathogens of PTO in the eligible patients were identified and analyzed. We hope our findings can help to update the database of PTO pathogens and thus inform the rational use of antibiotics in the acute stage of trauma in the departments of orthopedics and help to lower the incidence of PTO.
Methods
Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) aged ≥16 years and with a history of surgery for traumatic fractures of the limbs; (II) with clinical symptoms and signs, imaging data, or surgical pathology of the bone and soft tissue infection in the surgical area after surgery, which supported a diagnosis of chronic osteomyelitis; and (III) a positive finding in laboratory bacterial culture.
The exclusion criteria were as follows: (I) laboratory bacterial culture showed negative result although there was clear clinical evidence of infection (13); and (II) with a history of blood-borne osteomyelitis.
General data
A total of 5,268 patients with traumatic fractures of the limbs were treated in the orthopedics department of our hospital from January 1, 2012, to December 31, 2015. Among them 108 PTO cases (2.1%) were confirmed based on the clinical manifestations, imaging data, and surgical pathology. A total of 68 cases were transferred from other hospitals, among whom 53 cases were culture-positive; 39 patients were directly diagnosed in our hospital, among whom 31 cases were culture-positive. After 24 patients with negative cultures were ruled out, 84 patients (77.8%) entered the final analysis. There were 69 men and 15 women aged 16 to 72 years (mean: 43 years). The primary injuries were open in 66 cases (78.6%) and closed in 18 cases (21.4%).
This study was reviewed and approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical University, and all patients signed informed consent.
The clinical manifestations included the following: chills and fever (n=12); local skin redness, increased skin temperature, persistent pain, rebound tenderness, or deep tenderness (n=29); local swelling and pitting edema (n=42); poor incision healing, continuous exudation of fluid, or formation of a sinus tract (n=68); increased white blood cell (WBC) count (n=25); increased erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) (n=63); local liquid dark areas on X-ray and ultrasound (n=56); and liquid extracted by local puncture (n=46).
Time points of sample collection
For patients who had been using antibiotics, the antibiotics were discontinued and routine drug changes and topical treatment were applied, and a total of 72 patients underwent pathogen identification after 2 weeks (14,15). For patients who had not used antibiotics within the past 2 weeks, bacteria sampling was immediately performed. In our current series, bacterial sampling was carried out 23 to 482 days after fracture (mean: 135 days).
Procedure of sample collection
Specimen collection was performed by senior residents or attending doctors, during which skin disinfection was strictly performed to avoid exogenous bacterial contamination. For cases with suspected abscesses or liquid dark areas, percutaneous multi-point puncture was performed to extract fluid until the subperiosteal level or the deep bone was internally fixed regardless of whether there was a sinus tract or not; for patients with smaller liquid dark areas, the puncture needle was directed to the lesion under ultrasound. If the specimen collection by puncture failed and there was exudate from the sinus tract, the sinus secretions were collected for 3–5 consecutive days for bacterial culture. Fluids and tissue blocks were intraoperatively harvested at the surgical area for bacterial culture in all surgical patients.
Bacterial culture and antimicrobial susceptibility test
The puncture fluid, tissue blocks, or secretions were cultured separately in the microorganism laboratory. VITEK 2 Compact automatic bacterial identification and drug sensitivity analysis system (BioMerieux Inc., France) was used for bacterial identification and for setting the quality control strains, namely the gram-positive bacterial strains AST-GP6 (Staphylococcus aureus-ATCC29213) and AST-GP68 (Streptococcus pneumoniae-ATCC49619), along with the gram-negative bacterial strains, AST-GN13 (Escherichia coli-ATCC25922) and AST-GN09 (Pseudomonas aeruginosa-ATCC27853). The bacterial susceptibility data were determined through the M-100-S22 Protocol [Clinical & Laboratory Standards Institute® (CLSI) 2012, USA]. Methillin-resistant Staphylococcus aureus (MRSA) was detected by using the Kirby-Bauer test. Cefoxitin disc diffusion method was used for determining the heterogeneous populations of MRSA, and the results were judged according to the diameter of inhibition zone (DIZ).
Results
Infection sites in PTO patients
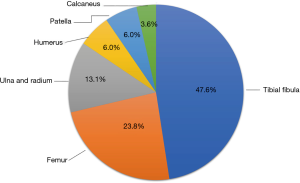
Of these 84 PTO patients, 40 had tibial and fibular fractures, 20 had femoral fractures, 11 had ulnar and radial fractures, 5 had humeral fractures, 5 had patella fractures, and 3 had calcaneal fractures. The tibial and fibular fractures were the most common fracture type, accounting for 47.6% of cases (Figure 1).
Bacterial isolates in PTO patients
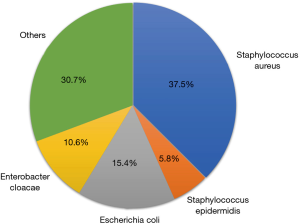
A total of 104 bacterial strains were detected in these 84 patients (Figure 2), of which 56 (53.85%) were gram-positive bacteria, which mainly included Staphylococcus aureus (n=39, 37.5%) and Staphylococcus epidermidis (n=6, 5.8%). Gram-negative bacteria were detected in 48 cases (46.15%), which mainly included Escherichia coli (n=16, 15.4%) and Enterobacter cloacae (n=11, 10.6%).
In addition, 66 patients were infected with a single bacterial strain (78.6%), and 18 were infected with multiple strains (21.4%). Staphylococcus aureus was the most common strain in patients infected by a single bacterial strain (31/66, 47.0%) or multiple strains (8/18, 44.4%) (Table 1).

Full table
Antimicrobial resistance among gram-positive bacteria
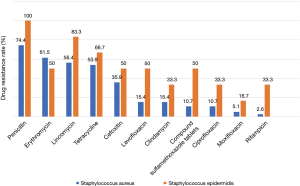
Gram-positive bacterial infections in this study mainly included Staphylococcus aureus and Staphylococcus epidermidis (43.3%). Both were sensitive to ampicillin, quinupristin, linazolamide, tigarycline, nitrofurantoin, and vancomycin. Staphylococcus aureus included MRSA in 17 cases and methicillin-sensitive Staphylococcus aureus (MSSA) in 22 cases. Staphylococcus aureus had the highest resistance to penicillin (up to 74.4%), followed by erythromycin, lincomycin, tetracycline, cefoxitin, oxacillin, levofloxacin, clindamycin, compound sulfamethoxazole tablets, ciprofloxacin, moxifloxacin, and rifampicin. Staphylococcus epidermidis also had the highest resistance to penicillin, followed by lincomycin, oxacillin, tetracycline, erythromycin, cefoxitin, levofloxacin, compound sulfamethoxazole tablets, clindamycin, ciprofloxacin, rifampicin, and moxifloxacin (Figure 3).
Antimicrobial resistance among gram-negative bacteria
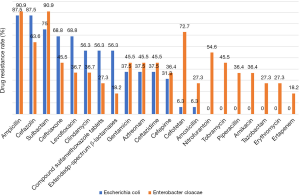
Gram-negative bacterial infections in our series mainly included Escherichia coli and Enterobacter cloacae (26.0%). Both were sensitive to streptomycin. Escherichia coli had the highest resistance to ampicillin and cefazolin (up to 87.5%), followed by sulbactam, ceftriaxone, levofloxacin, ciprofloxacin, compound sulfamethoxazole tablets, extended-spectrum β-lactamases, gentamicin, aztreonam, ceftazidime, and cefepime; it was least resistant to cefotetan and amoxicillin. Enterobacter cloacae was more resistant and had the highest resistance to sulbactam and ampicillin, followed by cefotetan, cefazolin, nitrofurantoin, ceftriaxone, gentamicin, aztreonam, ceftazidime, tobramycin, levofloxacin, ciprofloxacin, cefepime, piperacillin, amikacin, compound sulfamethoxazole tablets, amoxicillin, tazobactam, and erythromycin; it was least resistant to ertapenem and extended-spectrum β-lactamases (Figure 4).
Discussion
Commonly affected sites in PTO patients
In our current study, PTO affected the long bones in 76 patients (90.5%) and irregular bones in 8 cases (9.5%). Among them, the affected sites included long bones in the lower limbs in 60 cases (71.4%) and shoulder girdles in 16 cases (19.1%). These findings were similar to the historical data (2-year interval) in our area, and there was no obvious dynamic change, which may be due to the short interval between these 2 studies and the overlap of some cases. Although the fractures of irregular bones in the lower limbs had a relatively small proportion, they were associated with a more severe impact on patients’ functionality and quality of life than those involving long bones (13,16). Notably, since the operative time is often closely related to the occurrence of infections, it should be shortened as much as possible during trauma treatment in emergency settings (except for those treated according to the standard operating procedures) (17). Although the diagnosis of PTO depends on the clinical manifestations, imaging data, pathology, and bacterial culture, antibiotic prophylaxis may be needed to prevent chronic osteomyelitis in patients presenting with the clinical manifestations of early osteomyelitis, especially in those with traumatic fractures in the lower limbs. The principles of debridement for chronic osteomyelitis may refer to the criteria proposed by Xie et al. (2,13,16,18,19). After standardized specimen collection is performed, a diagnosis of PTO should be confirmed as early as possible based on clinical manifestations, laboratory results, and imaging findings (6,20).
Distribution of PTO-related bacterial strains at different time points and in different areas
A total of 104 strains were isolated from these 84 patients after the bacterial culture. Gram-positive bacteria were the most common species, accounting for 53.85% of cases; gram-negative bacteria were less common, accounting for 46.15%. Their positive rates decreased/increased by about 9% compared with the positive rates of Gram-positive bacteria (63.16%) and Gram-negative bacteria (36.84%) in our area 2 years ago. Staphylococcus aureus (37.5%) and Staphylococcus epidermidis (5.8%) remained the most dominant Gram-positive bacteria, although their positive rates decreased by about 10% and 2.1% when compared with the previous incidences. Among the Gram-negative bacteria, the positive rates of Escherichia coli and Enterobacter cloacae were 15.4% and 10.6%, which were about 0.3% lower and 3.5% higher than before, respectively (13). These data reflect the dynamic changes of PTO-related pathogens at different time intervals, which show no notable change in the ranking of bacterial isolates within a short period of time. As shown by comparisons with the results of a multi-center study in Beijing, the ranking of major Gram-positive bacteria was similar; however, the most dominant Gram-negative bacteria in Beijing was Pseudomonas aeruginosa (26.9%), followed by Escherichia coli (17.9%), showing significant difference from the positive rates in our area. Thus, the distribution of the main pathogens varied across different areas (2).
Compared with our previous study, the incidence of single infections decreased from 88.2% in the previous group to 78.6% in this group. The incidence of mixed infections reached 21.4% in our current study, which is an increase of nearly 10% from the previous study (11.7%) and might be explained by the long disease course, long-term irregular use of antibiotics, and transfer of previously treated patients from other hospitals. Nevertheless, the overall changes in the bacterial isolates and affected sites were not obvious in patients with mixed infections (13).
Changes in the spectrum of antimicrobial-resistant bacterial isolates
The most dominant bacterial isolates included Gram-positive strains (43.3%; including Staphylococcus aureus and Staphylococcus epidermidis) and Gram-negative strains (26.0%; including Escherichia coli and Enterobacter cloacae).
The Gram-positive bacteria were sensitive to ampicillin, quinupristin, linazolamide, tigarycline, nitrofurantoin, and vancomycin, which was consistent with the previous finding. However, our current study found that 25.6% of patients with Staphylococcus aureus infection were responsive to penicillin treatment (the rate of penicillin resistance was 74.4%), which was different from the data in the previous study, suggesting these new patients came from areas where relatively fewer antibiotics were used with the susceptible strain can lying dormant at the affected site for a long period of time (21). Therefore, for patients from remote areas with limited access to antibiotics, some conventional antibiotics can be used empirically after tissue sampling and before identification of drug-resistant bacteria. Escherichia coli, as the most dominant Gram-negative bacteria in our series, was sensitive to nitrofurantoin, tobramycin, piperacillin, amikacin, tazobactam, erythromycin, and ertapenem, which allowed the use of a wide range of antibiotics. In contrast, Staphylococcus epidermidis was sensitive only to streptomycin and was resistant to other antibiotics to varying degrees. Thus, for patients with confirmed Gram-negative bacterial infection, blind antibiotic prescription should be implemented cautiously before the result of a sensitivity analysis becomes available.
Methodological strength and limitations
As a single-center retrospective analysis, our current study was limited by its single sample source and small sample size. Also, it had limited power in reflecting the dynamic changes of PTO-related pathogens due to the short interval after the previous study; however, it authentically reflects the cross-sectional changes during the study period, and recent historical data can inform clinical practices.
To summarize, the incidence of PTO in the Zunyi area is similar to the national level. The most common site of infection was the lower extremity. Bacterial infections (mainly infection caused by a single bacterial type) were observed in 77.8% of the cases. Staphylococcus aureus was the most common pathogenic bacteria, followed by E. coli and Enterobacter cloacae. The antibiotic-resistant bacteria had characteristic distributions. These findings are valuable for the diagnosis and empirical treatment of PTO and the empirical use of antibiotics in grassroots hospitals in our area.
Acknowledgments
Funding: National Natural Science Foundation of China Regional Science Foundation Project (81760400), Guizhou Provincial Science and Technology Program (QKHZC [2018] 2760).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was reviewed and approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical University (No. 201842940112220588), and all patients signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen XP, Shi YK, Qiu GX, et al. Surgery. Second edition. Beijing: People's Medical Publishing House, 2011:1057.
- Qiao L, Xia ZL, Liu J, et al. Bacterial spectrum characteristics and antibiotic resistanc patterns of posttraumatic chronic osteomyelitis: a multi-center retrospective study. Chinese Journal of Orthopaedic Trauma 2016;18:769-74.
- Wang ZN, Li MY, Zheng QJ. Prevention and early detection of methicillin-resistant Staphylococcus aureus in orthopedic ward. Chinese Journal of Orthopedics 2015;35:686-90.
- Aytaç S, Schnetzke M, Swartman B, et al. Posttraumatic and postoperative osteomyelitis: surgical revision strategy with persisting fistula. Arch Orthop Trauma Surg 2014;134:159-65. [Crossref] [PubMed]
- Kanakaris N, Gudipati S, Tosounidis T, et al. The treatment of intramedullary osteomyelitis of the femur and tibia using the Reamer-Irrigator-Aspirator system and antibiotic cement rods. Bone Joint J 2014;96-B:783-8. [Crossref] [PubMed]
- Stranix JT, Lee ZH, Bellamy J, et al. Indications for Plain Radiographs in Uncomplicated Lower Extremity Cellulitis. Acad Radiol 2015;22:1439-42. [Crossref] [PubMed]
- de Mesy Bentley KL, MacDonald A, Schwarz EM, et al. Chronic Osteomyelitis with Staphylococcus aureus Deformation in Submicron Canaliculi of Osteocytes: A Case Report. JBJS Case Connect 2018;8:e8. [Crossref] [PubMed]
- Calhoun JH, Manring MM, Shirtliff M. Osteomyelitis of the Long Bones. Semin Plast Surg 2009;23:59-72. [Crossref] [PubMed]
- Zhang T, Sun ZH, Zheng YF, et al. Ilizarov method associated with bone lengthening for treating infected nonunion of tibia with large defects. Chinese Journal of Orthopedics 2008;28:353-7.
- Zeng BF, Xie XT. Prevention and treatment of infection after internal fixation. Chinese Journal of Orthopedics 2011;31:90-4.
- Yokogawa N, Ishikawa M, Nishitani K, et al. Immunotherapy synergizes with debridement and antibiotic therapy in a murine 1-stage exchange model of MRSA implant-associated osteomyelitis. J Orthop Res 2018;36:1590-8. [Crossref] [PubMed]
- Kremers HM, Nwojo ME, Ransom JE, et al. Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Joint Surg Am 2015;97:837-45. [Crossref] [PubMed]
- Ren YL, Peng JC, Li ZD, et al. Regional characteristics of bacteria infections and antibiotic resistance in postoperative traumatic limb fractures. Chinese Journal of Orthopaedic Trauma 2016;18:226-32.
- Schäfer P, Fink B, Sandow D, et al. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis 2008;47:1403-9. [Crossref] [PubMed]
- Della Valle C, Parvizi J, Bauer TW, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am 2011;93:1355-7. [Crossref] [PubMed]
- Peng J, Ren Y, He W, et al. Epidemiological, Clinical and Microbiological Characteristics of Patients with Post-Traumatic Osteomyelitis of Limb Fractures in Southwest China: A Hospital-Based Study. J Bone Jt Infect 2017;2:149-53. [Crossref] [PubMed]
- Pollak AN, Jones AL, Castillo RC, et al. The relationship between time to surgical debridement and incidence of infection after open high-energy lower extremity trauma. J Bone Joint Surg Am 2010;92:7-15. [Crossref] [PubMed]
- Xie Z. The difficulties and challenges of the diagnosis and treatment of extremity trauma. China J Trauma 2015;31:289-93.
- Yuan Z, Hu YY. Therapeutic effects of anti-infective reconstituted xenogeneic bone on tibial osteomyelitis in rabbits. Chinese Journal of Orthopedics 2003;23:230-4.
- Friedman A, Balfour S, Reinus W, et al. Emergency Department Extremity Radiographs in the Setting of Pain Without Trauma: Are They Worth the Pain? J Emerg Med 2015;49:152-8. [Crossref] [PubMed]
- Libraty DH, Patkar C, Torres B. Staphylococcus aureus reactivation osteomyelitis after 75 years. N Engl J Med 2012;366:481-2. [Crossref] [PubMed]