
Effect of Xpert MTB/RIF on the treatment of multi-drug-resistant or rifampicin-resistant tuberculosis screened out from re-treatment pulmonary tuberculosis patients, a prospective cohort study
Introduction
Re-treatment pulmonary tuberculosis (PTB) is defined as patients who have previously been treated with chemotherapy for a period of one month or longer, including relapse, failure, and return after loss of follow-up during treatment. The World Health Organization (WHO) recommends that re-treatment PTB patients be treated with the category II regimen, which consists of 5 standard drugs (1); however, the effective therapeutic rate of this regimen varies from 50% to 90% depending on the region and re-treatment type (2,3). A high rate of drug resistance has been found in patients with re-treatment PTB, which is associated with poor outcomes (3,4).
According to the 2015 Global TB report, 21% of re-treatment patients had multi-drug- or rifampicin-resistant TB (MDR/RR-TB) in comparison with 3.9% of new cases (5). A Chinese study showed that MDR-TB was present in 4.8% and 26.3% of new and re-treatment TB cases, respectively (6). Updated WHO guidelines recommend that drug susceptibility testing (DST) can be performed in all re-treatment TB patients, and individualized anti-TB treatments, even MDR-TB regimens, should be given based on the local prevalence of drug resistance and DST results (7). However, traditional methods for mycobacterial culture and DST are complex and time-consuming, prolonging diagnosis and increasing the risk of improper treatment and transmission of drug-resistant Mycobacterium tuberculosis (Mtb) strains (8). Therefore, rapid screening of MDR/RR-TB among re-treatment patients is important for the administration of appropriate regimens. Gene Xpert MTB/RIF® (Cepheid, Sunnyvale, CA, USA) is a quick and simple molecular test for the detection of rifampicin-resistant (RR) TB (9), which is the representative MDR-TB, since resistance to rifampicin (RFP) typically correlates with resistance to isoniazid (INH) (10). Therefore, Xpert MTB/RIF has been endorsed by the WHO as an essential diagnostic tool for patients in any re-treatment category (11). Although a large number of studies have evaluated the usefulness of Xpert MTB/RIF in diagnosing PTB patients (12), limited data are available regarding the impact of Xpert MTB/RIF on treatment for re-treatment PTB patients (13).
The present study aimed to determine the advantages and benefits of the Xpert MTB/RIF assay in the treatment of MDR/RR-TB screened out from re-treatment PTB cases, and to build treatment patterns for MDR/RR-TB based on testing Xpert MTB/RIF in clinic practice.
Methods
Study design
From June 2017 to June 2018, we prospectively enrolled consecutive re-treatment PTB suspects at Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China. The re-treatment PTB diagnostic criteria were in accordance with the WHO guidelines and relied on information regarding clinical symptoms, X-ray, sputum test, histological observations, and lack of improvement following treatment with broad-spectrum antibiotics (14). Patients with known MDR-TB or HIV infection were excluded.
Patients were randomly assigned to two groups: the Xpert MTB/RIF group (in which patients were tested using the Xpert MTB/RIF assay and mycobacterial culture) and the Mycobacterial tuberculosis (MTB) culture group (in which patients were tested by mycobacterial culture only). In accordance with routine practice, sputum or bronchial alveolar lavage fluid (BALF) samples were collected. Specimens were subjected to routine acid-fast bacilli smear microscopy, mycobacterial culture, identification and drug susceptibility testing (DST) in authorized TB laboratories in Shanghai Pulmonary Hospital. Following positive rifampicin resistance results in the Xpert MTB/RIF group, anti-MDR-TB regimens were initiated. In the MTB culture group, patients underwent anti-MDR/RR-TB chemotherapy when the DST results indicated resistance to RFP with/or without INH. All data associated with the treatment of patients were recorded, including: identification number; age; gender; results of the Xpert MTB/RIF assay, smear microscopy, culture, and DST; date of sputum or BALF sample collection and results; treatment regimens; and date of chemotherapy initiation. The diagnostic and treatment process for the present study is shown in Figure 1. Xpert MTB/RIF assay was performed only once for patients in Xpert MTB/RIF group.
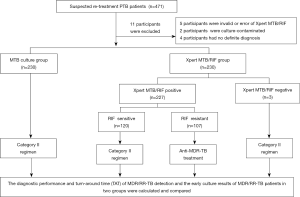
All participants provided written informed consent. The study was approved by the Institutional Review Board of Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China (Approval No. K16-298).
Definitions
The case definitions are in accordance with the National TB and WHO guidelines (1). “New cases” are defined as PTB patients who have never been given anti-TB drugs or have taken anti-TB drugs for a period of less than one month. “Re-treatment cases” include: (I) relapse (following prior completion of TB treatment); (II) treatment interruption (lost to follow-up during a prior treatment course); and (III) treatment failure (acid-fast bacilli sputum smear-positivity after a five-month treatment course or longer). “Multi-drug-resistant tuberculosis (MDR-TB)” refers to TB that is resistant to at least isoniazid and rifampicin. “Extensively drug-resistant tuberculosis (XDR-TB)” refers to TB that is resistant to at least isoniazid and rifampicin (like MDR-TB), any fluoroquinolone, and at least one of three second-line injectable anti-TB drugs, i.e., capreomycin, kanamycin, or amikacin.
Bacterial culture, DST, and Xpert MTB/RIF assays
Specimens were processed in N-acetyl-L-cysteine-NaOH-Na citrate, neutralized in phosphate-buffered saline (PBS), and centrifuged at 3,000 g for 15 min. The sediments were subsequently resuspended in 2 mL PBS and smears were stained with auramine O to detect acid-fast bacilli (AFB).
An aliquot of the processed specimen was placed in a mycobacterial growth indicator tube (MGIT) and bacterial culture was performed using the BD BACTEC™ MGIT™ 960 Mycobacteria Culture System (Becton Dickinson and Company, Allschwil, Switzerland). Incubation of cultures was carried out for 6 weeks or until signaled by the respective instrument as positive. Culture-based drug susceptibility testing (DST) was performed using the MODS assay (TB MODS KitTM, Hardy Diagnostics, Santa Maria, CA USA), following the manufacturer’s protocol.
The remaining specimens were subjected to the Xpert MTB/RIF assay, according to the manufacturer’s instructions (Cepheid, Sunnyvale, CA, USA). Specimens were transferred into a single-use disposable cartridge and placed in the GeneXpert™ Dx module. The semi-quantitative system automatically interpreted all results in less than 2 h, as very low, low, medium, or high. The output of rifampicin resistance was: detected, not detected, or indeterminate.
Statistical analysis
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated (plus 95% confidence intervals (CIs)) as compared with DST to evaluate the efficacy of Xpert MTB/RIF for the detection of MDR/RR-TB among re-treatment PTB patients. Clinical data consisted of the median and interquartile range (IQR) for age and time variables, and mean ± standard deviation (SD) for other continuous variables. χ2 test was employed for comparison of categorical variables, and an independent t-test and Mann-Whitney U test were used for continuous variables. P<0.05 was considered statistically significant. Statistical analysis was performed using SPSS 24.0 (IBM, Armonk, New York, USA).
Results
Study population
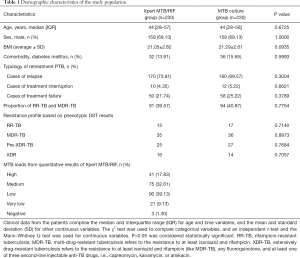
A total of 471 re-treatment PTB suspects were enrolled in the present study (Figure 1). Among these, 11 were excluded as a result of invalid Xpert MTB/RIF, culture contamination, or obscure diagnosis. Finally, 460 were included in the analysis. Patients were divided into two groups: 230 cases in the Xpert MTB/RIF group and 230 cases in the MTB culture group. The patient demographic characteristics are shown in Table 1. In the Xpert MTB/RIF group, the median age was 44 years old [IQR 28−57], 159 (69.13%) were male, the average BMI (body mass index) was 21.05±2.82, and 32 cases (13.91%) were in combination with diabetes mellitus; while in the MTB culture group, the median age was 44 years old [IQR 28−58], 159 (69.13%) were male, the average BMI was 21.29±2.61, and 36 cases (15.65%) were in combination with diabetes mellitus. All patients were HIV-negative. In the Xpert MTB/RIF group, 170 patients (73.91%) were “relapse”, 10 (4.35%) were “treatment interruption”, and 50 (21.74%) were “treatment failure”; while in the MTB culture group, 160 patients (69.57%) were “relapse”, 12 (5.22%) were “treatment interruption”, and 58 (25.22%) were “treatment failure”. The number of MDR/RR-TB cases was 91 (39.57%) in the Xpert MTB/RIF group and 94 (40.87%) in the MTB culture group. According to the Xpert MTB/RIF assay: the number of patients with high-level results was 41 (17.83%), those with medium-level was 75 (32.61%), those with low-level was 90 (39.13%), those with very low-level was 21 (9.13%), and those with negative results was 3 (1.30%) (Table 1).
Full table
Diagnostic value of Xpert MTB/RIF for screening out MDR/RR-TB among re-treatment PTB patients
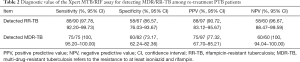
In the present study, a total of 157 cases with positive results from both Xpert MTB/RIF and MTB culture were included in the analysis. Conventional DST showed that there were 90 RR-TB patients among the 157 cases, and the Xpert MTB/RIF assay correctly detected 88 cases of these, with a sensitivity of 97.78%. A total of 13 patients (13/107; 12.15%) were resistant to RFP according to Xpert MTB/RIF and sensitive to isoniazid (INH) by phenotypic DST. Consequently, use of phenotypic DST as the reference standard showed that the PPV of Xpert MTB/RIF for the detection of RR-TB and MDR-TB among re-treatment PTB patients was 90.72% and 77.32%, respectively (Table 2).
Full table
Effect of the Xpert MTB/RIF assay on the treatment of MDR/RR-TB patients
The results of phenotypic DST were used as the reference standard for the initiation of anti-MDR-TB treatment regimens. In the present study, the turnaround time (TAT) from sample collection to the initiation of anti-MDR-TB treatment for MDR/RR-TB cases was significantly shorter in the Xpert MTB/RIF group, which was 1 [1−1] days vs. 52 [47−57] days in the MTB culture group (P<0.0001) (Table 3).

Full table
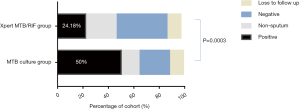
We compared the culture results between the two groups following two-month chemotherapy. The number of positive culture MDR/RR-TB patients in the Xpert MTB/RIF group was significantly reduced as compared with those in the MTB culture group (24.18% vs. 50.00%, P=0.0003) (Table 4 and Figure 2).

Full table
Discussion
In the present prospective cohort study, it was demonstrated that a diagnostic and treatment process involving the rapid molecular Xpert MTB/RIF assay could quickly and accurately detected MDR/RR-TB among re-treatment PTB patients, shortening the time to initiation of anti-MDR-TB treatment regimens for MDR/RR-TB patients, and reducing percentage of positive culture MDR/RR-TB patients following two-month chemotherapy.
The status of re-treatment PTB patients is more complicated as compared with newly diagnosed cases. Re-treatment patients have a history of chemotherapy, particularly if there has been previous-treatment interruption or failure, rendering it difficult to apply appropriate regimens. The WHO category II re-treatment regimen for all re-treatment PTB patients has had a low treatment success rate, which is unacceptable (15). Several studies have shown that fast molecular testing for diagnostic and treatment regimens can improve anti-TB treatment outcome (16,17). Individualized treatments based on drug sensitivity testing are more effective than category-based treatments (16).
Xpert MTB/RIF is an accurate and cost-effective automated PCR-based assay that detects Mycobacterium tuberculosis DNA and rifampicin resistance in less than 2 h. The sensitivity, specificity, PPV and NPV of Xpert MTB/RIF have been evaluated in suspected or newly diagnosed TB patients (11); however, few studies have examined the effects of the Xpert MTB/RIF assay on the treatment of patients with re-treatment PTB (18,19). Implementation of Xpert MTB/RIF in different health-care systems and settings would have different results, closely associated with its position in the clinical pathway and the way in which clinicians view the technology (20). Therefore, the present prospective study was carried out to determine the effect of Xpert MTB/RIF on the treatment of MDR/RR-TB in re-treatment PTB patients.
Positive RFP resistance according to the Xpert MTB/RIF assay suggests MDR-TB (9), and anti-MDR-TB therapy has been recommended by WHO for re-treatment PTB patients with Xpert MTB/RIF positive resistance to rifampicin (RFP). In the present study, among the patients who were positive for RFP resistance in the Xpert MTB/RIF assay, 12.15% (13/107) were INH-susceptible by phenotypic DST. A treatment regimen including isoniazid is beneficial for these patients. These results are similar to those reported by two other retrospective studies, in which 6.3–6.9% of the patients who were RFP-resistant according to Xpert MTB/RIF were INH-susceptible by DST (13,21). The positive predictive value (PPV) of the Xpert MTB/RIF assay for RR-TB was 90.72%, while that for MDR-TB was 77.32%. Thus, it can be concluded that RFP-resistance in re-treatment PTB patients according to the Xpert MTB/RIF assay indicates that anti-RR-TB regimens rather than anti-MDR-TB regimens should be performed. Moreover, rapid and accurate diagnostic tools for the detection of INH-resistant Mycobacterium tuberculosis deserve further study (13).
MDR/RR-TB patients in the Xpert MTB/RIF group had a much shorter interval for initiation of anti-MDR/RR-TB treatment; and following two-month chemotherapy, the percentage of positive culture MDR/RR-TB patients in the Xpert MTB/RIF group was significantly reduced. MDR/RR-TB transmission is a serious threat to public health. Introduction of Xpert MTB/RIF among re-treatment PTB patients has the potential to reduce MDR/RR-TB transmission by shortening the time to the initiation of MDR/RR-TB regimens. Thus, implementing the Xpert MTB/RIF assay in the treatment of re-treatment PTB patients has essential value in the clinic and in public health.
There exist certain limitations in the present study. There were 70 patients with positive Xpert MTB/RIF results but their culture results were negative. According to the WHO recommendation, a repeat Xpert MTB/RIF test is needed for these patients (22,23); however, due to various restrictions, only once Xpert MTB/RIF test was carried out in the present study. The treatment plans for these patients were based on the results of the Xpert MTB/RIF assay, and further clinical follow-up evaluation would be needed to evaluate the validity of the regimen. Moreover, since the present study was conducted in a single specialized tuberculosis hospital, a higher proportion of patients presented with MDR-TB, which may have had an impact on the prevalence of drug resistance.
Conclusions
In conclusion, through this prospective clinical study, the advantage of Xpert MTB/RIF on the treatment of MDR/RR-TB screened out from re-treatment PTB patients was demonstrated. Application of the Xpert MTB/RIF assay in the diagnosis and treatment among re-treatment PTB patients can significantly reduce the turn-around time for the initiation of anti-MDR/RR-TB treatment, quickly reducing the proportion of patients with positive MDR/RR-TB cultures, which is not only beneficial for patient treatment but also for reducing MDR-TB transmission. We recommend that re-treatment PTB patients receive anti-RR-TB chemotherapy following positive RFP resistance according to Xpert MTB/RIF.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All participants provided written informed consent. The study was approved by the Institutional Review Board of Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China (Approval No. K16-298).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Treatment of tuberculosis 3rd ed. Geneva: World Health Organization; 2003.
- Yoshiyama T, Shrestha B, Maharjan B. Risk of relapse and failure after retreatment with the Category II regimen in Nepal. Int J Tuberc Lung Dis 2010;14:1418-23. [PubMed]
- Ottmani SE, Zignol M, Bencheikh N, et al. Results of cohort analysis by category of tuberculosis retreatment cases in Morocco from 1996 to 2003. Int J Tuberc Lung Dis 2006;10:1367-72. [PubMed]
- Mak A, Thomas A, Del Granado M, et al. Influence of multidrug resistance on tuberculosis treatment outcomes with standardized regimens. Am J Respir Crit Care Med 2008;178:306-12. [Crossref] [PubMed]
- World Health Organization. WHO Global Tuberculosis Report 2016 Geneva Switzerland. 2016.
- Duan Q, Chen Z, Chen C, et al. The Prevalence of Drug-Resistant Tuberculosis in Mainland China: An Updated Systematic Review and Meta-Analysis. PLoS One 2016;11:e0148041. [Crossref] [PubMed]
- Noeske J, Voelz N, Fon E, et al. Early results of systematic drug susceptibility testing in pulmonary tuberculosis retreatment cases in Cameroon. BMC Res Notes 2012;5:160. [Crossref] [PubMed]
- World Health Organization. Tuberculosis profile-Bangladesh (high TB burden; high MDR-TB burden). 2010.
- Weyer K, Mirzayev F, Migliori GB, et al. Rapid molecular TB diagnosis: evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J 2013;42:252-71. [Crossref] [PubMed]
- World Health Organization. Rapid implementation of the Xpert MTB/RIF diagnostic test; technical and operational ‘how to’ practical consideration. 2011.
- Steingart KR, Schiller I, Horne DJ, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014.CD009593. [PubMed]
- Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax 2006;61:158-63. [Crossref] [PubMed]
- Kim YW, Seong MW, Kim TS, et al. Evaluation of Xpert((R)) MTB/RIF assay: diagnosis and treatment outcomes in rifampicin-resistant tuberculosis. Int J Tuberc Lung Dis 2015;19:1216-21. [Crossref] [PubMed]
- World Health Organization. Treatment of Tuberculosis: Guidelines. Geneva: 2010.
- Furin J, Gegia M, Mitnick C, et al. Eliminating the category II retreatment regimen from national tuberculosis programme guidelines: the Georgian experience. Bull World Health Organ 2012;90:63-6. [Crossref] [PubMed]
- Sun F, Li Y, Chen Y, et al. Introducing molecular testing of pyrazinamide susceptibility improves multidrug-resistant tuberculosis treatment outcomes: a prospective cohort study. Eur Respir J 2019;53. [Crossref] [PubMed]
- Lessells RJ, Cooke GS, McGrath N, et al. Impact of Point-of-Care Xpert MTB/RIF on Tuberculosis Treatment Initiation. A Cluster-randomized Trial. Am J Respir Crit Care Med 2017;196:901-10. [Crossref] [PubMed]
- Stagg HR, White PJ, Riekstina V, et al. Decreased Time to Treatment Initiation for Multidrug-Resistant Tuberculosis Patients after Use of Xpert MTB/RIF Test, Latvia. Emerg Infect Dis 2016;22:482-90. [Crossref] [PubMed]
- Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2014;383:424-35. [Crossref] [PubMed]
- Cox HS, Mbhele S, Mohess N, et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLoS Med 2014;11:e1001760. [Crossref] [PubMed]
- Trajman A, Durovni B, Saraceni V, et al. High positive predictive value of Xpert in a low rifampicin resistance prevalence setting. Eur Respir J 2014;44:1711-3. [Crossref] [PubMed]
- Falzon D, Jaramillo E, Schunemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011;38:516-28. [Crossref] [PubMed]
- World Health Organization. Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy Update. Geneva: 2013.


