
Moving trastuzumab emtansine (T-DM1) to the early setting of breast cancer treatment
Among women worldwide, breast cancer represents the most frequent malignancy and remains the leading cause of cancer-related death (1). Human Epidermal Growth Factor Receptor 2 (HER2)-positive disease accounts for approximately 20% of invasive breast neoplasms; in the absence of adequate targeted treatment, HER2-positivity leads to a more aggressive behavior and worse clinical outcomes as compared to other subtypes (2). With the introduction of anti-HER2 targeted therapy, the prognosis of patients with HER2-positive breast cancer has radically changed over the last 20 years in both the advanced and early settings (3).
Neoadjuvant systemic therapy for breast cancer has been originally conceived to treat patients with locally advanced and/or inoperable disease in order to improve chances and outcomes of surgical resections by downstaging the tumor and regional lymph nodes (4). Administering chemotherapy before surgery is associated with similar survival outcomes as when given in the adjuvant setting (5). However, the neoadjuvant approach has recently gained more attention considering the advantages of both allowing an in vivo assessment of tumor response and increasing the rates of conservative surgery but also because of the possibility to adapt the adjuvant treatment according to the results of the pathology report at the time of surgery (6). This is of particular relevance for patients with HER2-positive early breast cancer.
Chemotherapy (consisting in taxane-based regimens with or without the use of anthracyclines) plus anti-HER2 targeted treatment with trastuzumab and pertuzumab is the current most effective available neoadjuvant therapy in patients with HER2-positive early breast cancer (7,8). Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate composed of the humanized monoclonal antibody trastuzumab covalently linked to a cytotoxic agent (i.e., a tubulin inhibitor). The efficacy of T-DM1 has been consistently demonstrated in the metastatic setting both in the earlier and later lines of therapy with improvement in both progression-free survival and overall survival (OS) as well as a favorable safety profile (9,10). Based on its efficacy and safety in patients with metastatic breast cancer, several studies have been conducted more recently for moving T-DM1 to the early setting of breast cancer treatment.
The KRISTINE study is one of the largest randomized trials that investigated the role of T-DM1 as neoadjuvant therapy in patients with HER2-positive early breast cancer (11,12). A total of 444 patients were randomized to neoadjuvant chemotherapy plus dual anti-HER2 blockade (docetaxel, carboplatin, trastuzumab, pertuzumab – TCH+P) or to a “chemotherapy-free” regimen with T-DM1 plus pertuzumab (T-DM1+P). Patients randomly allocated to the TCH+P arm received neoadjuvant chemotherapy plus dual anti-HER2 blockade for 6 cycles and continued trastuzumab plus pertuzumab alone during the adjuvant phase, whereas patients randomized to the T-DM1+P arm received T-DM1 and pertuzumab during both the neo-adjuvant and adjuvant phases of the study. Neoadjuvant and adjuvant anti-HER2 targeted therapy was given every 3 weeks for 18 cycles in both study arms. Among the 444 patients enrolled, median age was 50 years, 167 (38%) had hormone receptor-negative disease. The majority of the patients (369, 83%) had baseline stage IIA–IIIA, while the remaining 75 patients (17%) presented with baseline IIIB– IIIC stage. As reported in the first analysis, use of T-DM1+P resulted in a significantly lower rate of pathologic complete response (pCR) as compared to TCH+P (44% vs. 56%, absolute difference −11.3%; P=0.016). Patients with hormone-receptor negative disease had higher probability to achieve a pCR regardless of the therapy performed (73% and 54% with TCH+P and T-DM1+P, respectively), while in those with hormone-receptor positive tumors, the rates of pCR were 44% and 35% with TCH+P and T-DM1+P, respectively (11). In the Journal of Clinical Oncology, Sara A. Hurvitz and colleagues have recently reported updated results of the KRISTINE trial with the secondary endpoints event-free survival (EFS), invasive disease-free survival (IDFS), safety and patient-reported outcomes (12). At a median follow-up of 37 months, as compared with TCH+P, the use of T-DM1+P was associated with a higher risk of an EFS event (HR 2.61; 95% CI: 1.36–4.98), but a similar risk of an IDFS event (HR 1.11; 95% CI: 0.52–2.40). During the overall study period, serious adverse events (SAEs) were less frequent with T-DM1+P compared to TCH+P. This was driven by the better safety profile of T-DM1+P in the neoadjuvant phase; on the contrary, T-DM1+P during the adjuvant phase was associated with higher number of patients developing a SAE as compared to adjuvant trastuzumab plus pertuzumab. In the T-DM1+P arm, the most frequent SAE was anemia (5.8%) followed by neutropenia (3.6%), peripheral neuropathy (3.1%), and decreased platelet count (2.2%). In the TCH+P arm, neutropenia (25.1%), diarrhea (15.5%), febrile neutropenia (15.1%), and anemia (11%) were the most frequent SAEs. Analysis of the patient-reported outcomes showed that patients receiving T-DM1+P reported fewer worsening scores in their quality of life during the neoadjuvant phase compared to patients receiving TCH+P, while similar scores were observed during the adjuvant phase. Regardless of treatment arm, pCR was associated with a reduced risk of an IDFS event (HR 0.24; 95% CI: 0.09–0.60) (12).
Despite being a negative study, the results from the KRISTINE trial raise some important considerations for improving the management of patients with HER2-positive early breast cancer, in terms of further increasing our understanding on the value of obtaining a pCR, the biology of HER2-positive tumors according to hormone receptor status and how to optimize T-DM1 use in the early setting.
The rate of pCR is the primary endpoint in most of the neoadjuvant trials since many years based on the several available studies suggesting that pCR correlates with patients’ outcomes. As show in the CTNeoBC pooled analysis, achievement of a pCR is associated with better EFS (HR 0.48; 95% CI: 0.43–0.54) and OS (HR 0.36; 95% CI: 0.31–0.42) than in the case of residual disease at surgery (13). Considering the prognostic value of pCR according to breast cancer subtype, the strongest association with long-term outcomes was observed in patients with the aggressive forms of breast cancer including the HER2-positive population, mainly in the presence of hormone receptor-negative status (13).
A growing amount of evidence suggests that the presence or absence of hormone receptors distinguish two different subtypes of HER2-positive breast cancer characterized by a distinct natural history (14). Many neoadjuvant trials including the KRISTINE study have shown major differences in the rates of pCR according to hormone receptor status with higher chances for patients with hormone receptor-negative/HER2-positive breast cancer (15). This important issue may highlight the need to develop different and ad hoc therapeutic strategies for patients with these different tumor subtypes. Although most of the trials in the early setting of HER2-positive disease are still being conducted including all breast cancer patients irrespective of hormone receptor status, the West German Study Group has recently tried to provide precise answers on this regard with the ADAPT trial (16). In this study, patients with hormone-receptor positive/HER2-positive early breast cancer were randomized to receive neoadjuvant T-DM1 (with or without endocrine therapy) or trastuzumab plus endocrine therapy. Neoadjuvant anti-HER2 targeted therapy was administered for 4 cycles every 3 weeks. The rate of pCR was 41.0% in the T-DM1 arm, 41.5% in the T-DM1 plus endocrine therapy arm, and 15.1% in the trastuzumab plus endocrine therapy (P<0.001) (16). In the KRISTINE study, pCR was achieved by around 35% of patients with hormone receptor-positive/HER2-positive disease treated with T-DM1+P (11). These results support the hypothesis of de-escalating treatment with “chemotherapy-free” strategies in selected patients. However, appropriate patient selection is essential and needs to be further investigated in the future.
The most important data so far with the use of T-DM1 in HER2-positive early breast cancer comes from the post-neoadjuvant setting in patients not achieving a pCR after neoadjuvant chemotherapy plus trastuzumab with or without pertuzumab. The KATHERINE study has recently led to the approval of T-DM1 as adjuvant treatment in these patients (17). In the trial, patients with residual disease after standard neoadjuvant taxane-based chemotherapy (with or without anthracyclines) plus trastuzumab (with or without pertuzumab) were randomized to T-DM1 or standard trastuzumab to complete 1 year of anti-HER2 therapy. A total of 72.3% of the 1486 patients enrolled had hormone receptor positive-disease; an anthracycline-containing neoadjuvant chemotherapy regimen was given to the majority of the patients (76.9%) and 18% of them received dual anti-HER2 blockade with trastuzumab and pertuzumab. IDFS was significantly improved among patients who received T-DM1 compared to trastuzumab (HR 0.50; 95% CI: 0.39–0.64, P<0.001). The benefit of adjuvant T-DM1 was demonstrated in both patients with hormone receptor-positive and hormone receptor-negative disease (17). Therefore, T-DM1 should now be considered the standard adjuvant anti-HER2 treatment in patients not achieving a pCR after neoadjuvant chemotherapy plus anti-HER2 therapy.
The KATHERINE trial has completely changed the treatment algorithm for managing patients with HER2-positive early breast cancer by favoring even more the use of the neoadjuvant approach in most of these cases. The only exception is represented by patients with stage I disease (tumors ≤2 cm and node negative) for whom the APT trial has shown very good outcomes with a de-escalated approach including weekly paclitaxel and trastuzumab (18). At a median follow-up of 6.5 years, 7-year DFS was 93% (95% CI: 90.4–96.2) and 7-year OS was 95% (95% CI: 92.4–97.7). In patients with hormone receptor-positive tumors, the 7-year DFS was 94.6% (95% CI: 91.8–97.5) while among patients with hormone receptors-negative tumors, 7-year DFS was 90.7% (95% CI: 84.6–97.2) (18). A further step in de-escalating the treatment of these patients has been more recently investigated in the ATEMPT trial (19). Women with histologically confirmed HER2-positive breast tumors ≤2 cm without nodal involvement were randomized to receive T-DM1 for 17 cycles every 3 weeks or standard paclitaxel plus trastuzumab for 12 weeks (followed by trastuzumab to complete 1 year of treatment). A total of 43% of the patients enrolled were diagnosed with a tumor size smaller than 1 cm and the majority (75%) had a positive hormone receptor status. Overall, 3-year DFS was 97.7% (95% CI: 96.2–99.3%) in the T-DM1 arm with only 2 of the 383 patients enrolled experiencing a distant recurrence. Notably, 23.5% of the patients discontinued treatment, being toxicity the most important reason (17.0% of the patients) (19). Although these are promising results to move a “chemotherapy-free” regimen as the only adjuvant treatment in selected patients with low-risk HER2-positive breast cancer, longer follow-up is needed. Financial toxicity is another important issue to be considered before allowing widespread use of T-DM1 in this setting.
Based on the availability of all these new important data including with the use of T-DM1 in the early setting, the management of patients with HER2-positive early breast cancer has radically changed (Figure 1). Clinical stage at presentation is crucial to define the best approach. Patients with tumors ≤2 cm without nodal involvement are candidates to surgery followed by a de-escalated strategy with weekly paclitaxel and trastuzumab. All the other patients (with node-positive and/or tumors >2 cm) should now be candidates to neoadjuvant chemotherapy (with a taxane-based regimen with or without anthracyclines) plus anti-HER2 therapy including trastuzumab plus, if available, pertuzumab. The neoadjuvant approach would help clarifying the best adjuvant treatment being the continuation of trastuzumab alone (or plus pertuzumab, if available, in the presence of high-risk features at diagnosis) to complete 1 year in patients achieving pCR and switching to T-DM1 in those with residual disease at surgery.
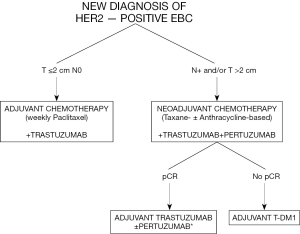
Despite major improvement in the care of patients with HER2-positive early breast cancer, some unanswered questions remain in this field including the possibility to de-escalate chemotherapy burden in a higher number of patients (20) as well as to escalate treatment approaches in the presence of poor prognostic features (21). More attention should be also paid to the management of patients with hormone receptor-positive/HER2-positive disease (22), including the optimization of the best adjuvant endocrine treatment approach (23-25). Ongoing and future studies are awaited to further improve the care of patients with HER2-positive early breast cancer. More personalized approaches will lead to a continuous improvement in survival rates but at the same time to a reduction in treatment burden.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Matteo Lambertini served as consultant for Roche and Lilly, and received speaker’s honoraria from Theramex, Roche and Takeda outside the submitted work. The other authors declare no conflict of interest.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Lambertini M, Pondé NF, Solinas C, de Azambuja E. Adjuvant trastuzumab: a 10-year overview of its benefit. Expert Rev Anticancer Ther 2017;17:61-74. [Crossref] [PubMed]
- Brandão M, Pondé NF, Poggio F, et al. Combination therapies for the treatment of HER2-positive breast cancer: current and future prospects. Expert Rev Anticancer Ther 2018;18:629-49. [Crossref] [PubMed]
- Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007.CD005002. [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- Caparica R, Lambertini M, Pondé N, et al. Post-neoadjuvant treatment and the management of residual disease in breast cancer: state of the art and perspectives. Ther Adv Med Oncol 2019;11:1758835919827714. [Crossref] [PubMed]
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:1674. [Crossref] [PubMed]
- Denduluri N, Chavez-MacGregor M, Telli ML, et al. Selection of Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36:2433-43. [Crossref] [PubMed]
- Cardoso F, Senkus E, Costa A, et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)†. Ann Oncol 2018;29:1634-57. [Crossref] [PubMed]
- Giordano SH, Temin S, Chandarlapaty S, et al. Systemic Therapy for Patients With Advanced Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36:2736-40. [Crossref] [PubMed]
- Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2018;19:115-26. [Crossref] [PubMed]
- Hurvitz SA, Martin M, Jung KH, et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study. J Clin Oncol 2019;37:2206-16. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Lambertini M, Campbell C, Gelber RD, et al. Dissecting the effect of hormone receptor status in patients with HER2-positive early breast cancer: exploratory analysis from the ALTTO (BIG 2-06) randomized clinical trial. Breast Cancer Res Treat 2019;177:103-14. [Crossref] [PubMed]
- Vaz-Luis I, Winer EP, Lin NU. Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes? Ann Oncol 2013;24:283-91. [Crossref] [PubMed]
- Harbeck N, Gluz O, Christgen M, et al. De-Escalation Strategies in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Early Breast Cancer (BC): Final Analysis of the West German Study Group Adjuvant Dynamic Marker-Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy Response Prediction in Early BC HER2- and Hormone Receptor-Positive Phase II Randomized Trial-Efficacy, Safety, and Predictive Markers for 12 Weeks of Neoadjuvant Trastuzumab Emtansine With or Without Endocrine Therapy (ET) Versus Trastuzumab Plus ET. J Clin Oncol 2017;35:3046-54. [Crossref] [PubMed]
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019;380:617-28. [Crossref] [PubMed]
- Tolaney SM, Guo H, Pernas S, et al. Seven-Year Follow-Up Analysis of Adjuvant Paclitaxel and Trastuzumab Trial for Node-Negative, Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer. J Clin Oncol 2019;37:1868-75. [Crossref] [PubMed]
- Tolaney SM, Trippa L, Barry W, et al. Abstract GS1-05: TBCRC 033: a randomized phase II study of adjuvant trastuzumab emtansine (T-DM1) vs paclitaxel (T) in combination with trastuzumab (H) for stage I HER2-positive breast cancer (BC) (ATEMPT). Cancer Res 2020;80:GS1-05.
- Lambertini M, Poggio F, Bruzzone M, et al. Dose-dense adjuvant chemotherapy in HER2-positive early breast cancer oatients before and after the introduction of trastuzumab: exploratory analysis of the GIM2 trial. Int J Cancer 2019. [Crossref] [PubMed]
- Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol 2019;11:1758835919833519. [Crossref] [PubMed]
- Guarneri V, Dieci MV, Bisagni G, et al. De-escalated therapy for HR+/HER2+ breast cancer patients with Ki67 response after 2-week letrozole: results of the PerELISA neoadjuvant study. Ann Oncol 2019;30:921-6. [Crossref] [PubMed]
- Bartlett JMS, Ahmed I, Regan MM, et al. HER2 status predicts for upfront AI benefit: A TRANS-AIOG meta-analysis of 12,129 patients from ATAC, BIG 1-98 and TEAM with centrally determined HER2. Eur J Cancer 2017;79:129-38. [Crossref] [PubMed]
- Dackus GMHE, Jóźwiak K, Sonke GS, et al. Optimal adjuvant endocrine treatment of ER+/HER2+ breast cancer patients by age at diagnosis: A population-based cohort study. Eur J Cancer 2018;90:92-101. [Crossref] [PubMed]
- Lambertini M, Blondeaux E, Perrone F, et al. Improving Adjuvant Endocrine Treatment Tailoring in Premenopausal Women With Hormone Receptor–Positive Breast Cancer. J Clin Oncol 2019. [Crossref] [PubMed]


