A two-way comparison of whole-body 18FDG PET-CT and whole-body contrast-enhanced MRI for distant metastasis staging in patients with malignant tumors: a meta-analysis of 13 prospective studies
Introduction
For patients with malignant tumors, the effective detection of distant metastasis makes a vital contribution to their prognosis. The TNM staging system informs the classification of most malignant tumors, and when the presence of distant metastasis is confirmed, the patient’s course of therapy and prognosis are decided. Accurate staging of distant metastasis is crucial for choosing adequate oncological therapy. With the simultaneous acquisition of functional and anatomical datasets, 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18FDG PET-CT) could provide a precise method by which to assess distant metastasis staging for a range of different cancers, especially those affecting the head and neck, lungs, breasts, and the digestive system (1). Due to improvements in sequencing, multi-channel technology, movable table platforms, and multiplanar reconstruction, magnetic resonance imaging (MRI) is increasingly being used in the whole-body staging of distant metastasis (2). Our previous meta-analysis of 9 studies with data on a per-patient analysis of 1,070 patients demonstrated that 18FDG PET-CT and contrast-enhanced MRI performed comparably as detectors of distant metastasis in patients with malignant tumors (2). Moreover, more prospective studies of 18FDG PET-CT and contrast-enhanced MRI for the staging of distant metastasis in various types of cancer have been reported (3-6). We carried out an updated meta-analysis to produce an evaluation and comparison of 18FDG PET-CT and contrast-enhanced MRI through the analysis of studies that focused on both diagnostic methods for the same patients with malignant tumors.
Methods
Literature search
Digital literature searches of the MEDLINE and Embase databases were performed to collect relevant published articles comparing the performances of whole-body 18FDG PET-CT and whole-body contrast-enhanced MRI in assessing distant metastasis in same cancer patients. Our searches were based on combinations of the following terms: MRI, magnetic resonance imaging, PET, positron emission tomography, staging, distant metastasis, and distant recurrence. We searched for articles published in the period from December 31, 1950 to August 1, 2019. We screened the references of the articles that were initially returned in our searches to identify any other research of potential interest. When information vital for our meta-analysis was not included in the relevant studies, we made direct contact with the authors for further data. We applied no restrictions on language in our search and selection of relevant studies.
Study selection
Studies were included in our meta-analysis if the following criteria were met: (I) whole-body 18FDG PET-CT and whole-body contrast-enhanced MRI were both used to evaluate distant metastasis on the same patients with malignant tumors; (II) distant metastasis was confirmed by the histology and imaging follow-up data; (III) analysis was carried out on a per-patient basis; (IV) the study was based on a minimum sample size of 20 patients; (V) in cases where the same data or subsets of data were repeated, the study with the larger sample size was considered for inclusion; (VI) the whole-body MRI must have included basic sequences of T1 and contrast enhanced T1. T2 sequence and short-time inversion recovery (STIR) sequence may be used as the selective sequences. Studies were not included if they met the following criteria: (I) the totals of true positives, false positives, true negatives, and false negatives were not included; (II) only contrast-enhanced MRI or 18FDG PET-CT was performed; (III) the diffusion-weighted imaging (DWI) sequence was carried out for whole-body MRI; (IV) non-original articles (conference abstracts, comments, letters to the editors, reviews, etc.).
Data extraction and quality assessment
Data was extracted from the relevant studies by two reviewers independently extracted the relevant. In the case of disputes, a consensus was reached through discussion. The following data was recorded: first author’s name; the study’s publication year and country; patient sample size; patient characteristics including age, sex, and primary tumor locations; the technical protocols of contrast-enhanced MRI and 18FDG PET-CT; reference standard; and study results (number of true positives, false positives, true negatives, and false negatives).
Two reviewers conducted independent assessments of the methodological quality of the studies using “Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2” (7). This updated tool assesses risk of bias and applicability concerns in four core areas: patient selection, index test, reference standard, and flow and timing). Each of these was assessed as low, high, or unclear risk.
Statistical analysis
All analyses were conducted with Stata version 11.0 (Stata Corporation, TX, USA). A bivariate model was used to calculate weighted overall estimates for sensitivity, specificity, diagnostic odds ratios (DORs), positive likelihood ratios (PLRs), and negative likelihood ratios (NLRs) with 95% confidence interval (CI) as our primary outcome measures. We then constructed summary receiver operating characteristic (SROC) curves for 18FDG PET-CT and contrast-enhanced MRI, respectively (8-10).
This analysis included data regardless of the locations of the primary tumor. The heterogeneity among the different studies for 18FDG PET-CT and contrast-enhanced MRI was analyzed and assessed by a χ2 test and I2 statistics (11-12). Heterogeneity was considered to exist when I2>50% and P<0.05 (11-12).
We used our pooled sensitivity and specificity estimates to work out the negative predictive values of 18FDG PET-CT and contrast-enhanced MRI when distant metastasis was assumed to have a prevalence rate of 10%, 20%, and 30%, respectively (10).
We also performed subgroup analyses for 18FDG PET-CT and contrast-enhanced MRI according to locations of the primary tumor (head and neck cancer vs. lung cancer) (8-10).
The publication bias was evaluated using Deeks’ funnel plot (13), which was conducted by means of a regression test of the natural logarithm of the DOR on the inverse of the adequate sample size. The presence of publication bias was indicated by asymmetry in the funnel plot, with P<0.05 indicating significant asymmetry.
Results
Study selection and description
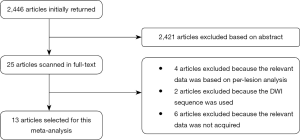
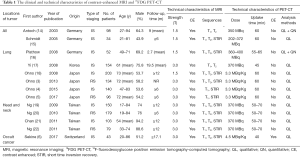
The flow diagram of the systematic literature review is shown in Figure 1. After being independently reviewed, 13 articles involving 1,465 patients (3-6,14-22) were deemed eligible for inclusion in this meta-analysis (Table 1). The study sample sizes ranged from 34 to 203 patients (median, 103 patients). In 1 study (43 patients), only occult cancer patients were enrolled. In 2 studies (132 patients) patients with various cancers were included. In 4 studies (511 patients), only patients with head and neck cancer were included. In 6 studies (779 patients), only lung cancer patients were enrolled. All 13 studies stated that they were conducted prospectively.
Full table
Study quality
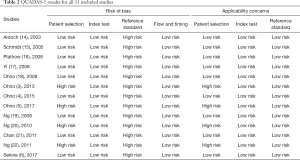
The quality assessment results conducted using QUADAS-2 are presented in Table 2. The risk of bias concerning patient selection was low in 9 of the 13 studies. Each of the studies had a low risk of bias in relation to the index test. These results were interpreted independently from the reference standard results. However, risk of bias in each of the 13 studies was high in relation to the reference standard as the reference standard was not carried out independently from the index test results.
Full table
Accuracy of contrast-enhanced MRI and 18FDG PET-CT
Summary of sensitivity, specificity, PLR, and NLR estimates
The weighted overall estimates of sensitivity, specificity, PLR, and NLR for 18FDG PET-CT and contrast-enhanced MRI were 0.84 (95% CI, 0.72 to 0.91) and 0.85 (95% CI, 0.73 to 0.93), 0.96 (95% CI, 0.94 to 0.97) and 0.98 (95% CI, 0.96 to 0.99), 18.8 (95% CI, 13.9 to 25.4) and 55.1 (95% CI, 19.4 to 157.0), and, 0.17 (95% CI, 0.10 to 0.30) and 0.15 (95% CI, 0.08 to 0.29), respectively (Table 3). This study confirmed that significant heterogeneity existed in 18FDG PET-CT groups (I2=0.81; P<0.05), and no heterogeneity existed in contrast-enhanced MRI groups (I2=0; P>0.05).

Full table
The performance comparison of 18FDG PET-CT and contrast-enhanced MRI in all of the 13 comparative studies (1,465 patients) suggested no differences in sensitivity or specificity between 18FDG PET-CT and contrast-enhanced MRI (P>0.05).
SROC curves
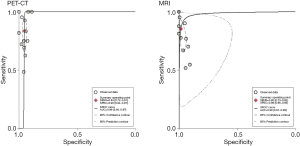
The SROC curves for 18FDG PET-CT and contrast-enhanced MRI from each of the 13 comparative studies are displayed in Figure 2. The overall weighted area under the SROC curves for 18FDG PET-CT and contrast-enhanced MRI was 0.96 (95% CI, 0.94 to 0.97) and 0.98 (95% CI, 0.97 to 0.99), respectively.
Negative predictive values
With an assumed distant metastasis prevalence of 10%, 20%, and 30% on a per-patient basis, the NPVs of 18FDG PET-CT were 0.98, 0.96, and 0.94, respectively, and those for contrast-enhanced MRI were 0.98, 0.96, and 0.94, respectively.
Publication bias
In both the 18FDG PET-CT (P=0.01>0.05) and the contrast-enhanced MRI groups (P=0.20>0.05) there was no significant publication bias identified. Each group had a symmetrical funnel plot, indicating publication bias to be statistically insignificant.
Subgroup analysis
Head and neck cancer
Four studies enrolled patients with tumours in the head and neck and performed both 18FDG PET-CT and contrast-enhanced MRI on a total of 511 patients. The weighted overall estimates in relation to sensitivity, specificity, PLR, and NLR for 18FDG PET-CT and contrast-enhanced MRI in patients with head and neck cancer were 0.82 (95% CI, 0.69 to 0.90) and 0.81 (95% CI, 0.64 to 0.90), 0.97 (95% CI, 0.94 to 0.98) and 0.98 (95% CI, 0.95 to 0.99), 23.9 (95% CI, 14.1 to 40.6) and 36.5 (95% CI, 13.7 to 97.5), and, 0.19 (95% CI, 0.11 to 0.34) and 0.20 (95% CI, 0.10 to 0.39), respectively (Table 3). This suggests 18FDG PET-CT had similar sensitivity and specificity compared with contrast-enhanced MRI (Table 3).
Lung cancer
Six studies enrolled only lung cancer patients and performed both 18FDG PET-CT and contrast-enhanced MRI on a total of 779 patients. The weighted overall estimates in relation to sensitivity, specificity, PLR, and NLR for 18FDG PET-CT and contrast-enhanced MRI in patients with lung cancer were 0.72 (95% CI, 0.53 to 0.85) and 0.83 (95% CI, 0.62 to 0.94), 0.95 (95% CI, 0.93 to 0.96) and 1.00 (95% CI, 0.86 to 1.00), 13.5 (95% CI, 9.4 to 19.5) and 400.8 (95% CI, 4.8 to 33130.6), and, 0.30 (95% CI, 0.17 to 0.53) and 0.17 (95% CI, 0.07 to 0.42), respectively. This suggests that there were no major differences between contrast-enhanced MRI and 18FDG PET-CT, although the point estimates suggest that both specificity and sensitivity improved by 5-11% when contrast-enhanced MRI was used (Table 3).
Discussion
The correct staging of distant metastasis is crucial for cancer patients, as both prognosis and course of therapy are highly dependent on TNM stage. The concept of oncological whole-body imaging is not new; however, the possibility of routinely applying the procedure in a clinical setting is limited. Whole-body PET-CT and whole-body MRI are now increasingly used to replace the earlier multimodal staging strategies, but the findings between these two diagnostic modalities for distant metastasis are incongruent. In this updated meta-analysis of 13 studies (1,465 patients), we calculated summary estimates for 18FDG PET-CT and contrast-enhanced MRI, and SROC curves were also constructed. This meta-analysis documents that 18FDG PET-CT and contrast-enhanced MRI had high sensitivity (0.84 and 0.85) and specificity (0.96 and 0.98) for the detection of distant metastasis.
In cancer diagnosis, the negative predictive value acts as a a single indicator of the probability of distant metastasis being present in cases where the diagnostic result is negative (10). In this meta-analysis, if the prevalence of distant metastasis was assumed to be 10% and 20%, the negative predictive values for 18FDG PET-CT/contrast-enhanced MRI were 0.98/0.98 and 0.96/0.96. At tumor staging, when the prevalence of distant metastasis was assumed to be 10–20%, 18FDG PET-CT and contrast-enhanced MRI could provide valuable information, with the probability of distant metastasis being reduced to as low as 2–4% in cases of negative diagnostic results.
Contrast-enhanced MRI showed higher sensitivity than 18FDG PET-CT in relation to lung cancer than in head and neck cancer (0.13 vs. 0.01). The reason for this phenomenon is that contrast-enhanced MRI and 18FDG PET-CT have different advantageous qualities for detecting distant metastatic lesions in different organs. For patients with cancer of the head and neck, the skeleton and lungs are common sites for distant metastasis, accounting for 80% to 90% of cases of distant metastasis (16-19). Previous studies found contrast-enhanced MRI to have similar sensitivity to 18FDG PET-CT in detecting bone and lung metastasis (14,23). The liver and brain are common distant-sites for lung cancer patients, involving 40% to 50% of patients with distant metastasis (3-5,13-15). Compared with 18FDG PET-CT, contrast-enhanced MRI showed higher sensitivity in detecting metastasis in the brain and liver, because some metastatic lesions may be obscured by high physiological FDG uptake of brain and liver (24,25). For the detection of brain metastasis in particular, the sensitivity for 18FDG PET-CT is only 21%, but for contrast-enhanced MRI it is 77% (25). Contrast-enhanced brain MRI could also offer more accurate diagnosis of metastatic brain lesions than 18FDG PET-CT. Another study showed that the combination of 18FDG PET-CT and brain MRI may enhance the sensitivity of only 18FDG PET-CT (89.6% versus 83.0%) for distant metastasis staging in patients with lung adenocarcinoma (26). Therefore, the inclusion of the brain in the procedure for whole-body MRI is another advantage it holds over PET/CT.
Moreover, the limited sensitivities of 18FDG PET-CT imaging in patients with primary hepatocellular carcinomas, carcinomas of the gastrointestinal tract, renal cell carcinoma, pheochromocytomas, and many neuroendocrine tumors of the pancreas have also been identified (27-30). Another disadvantage of 18FDG PET-CT is its low r dosage of radiation, which amounts to about 22 mSv for the PET-CT for the area from the head to the thighs (14).
The joint anatomical and functional capabilities of MRI may provide new insight for distant metastasis staging in patients with malignant tumors. Ohno et al. (18) stated that sensitivity and specificity of whole-body MRI without DWI for M1 staging of lung cancer was 60%, and 92% of whole-body MRI with DWI was 70.0% and 92.0%, respectively. The addition of DWI could improve the sensitivity of whole-body MRI for distant metastasis staging. This meta-analysis involved studies with basic sequences of T1 and contrast-enhanced T1 for whole-body MRI. Due to the limited number of studies with a DWI sequence, we excluded whole-body MRI studies in which the DWI sequence was used. Large, multicenter, and prospective studies which involve strict standardization of MRI protocols remain essential for evaluating the diagnostic capacity of whole-body MRI with DWI as a tool for effective distant metastasis staging in cancer patients. Until recently, the availability of whole-body MRI has been low. Negative aspects in terms of its practicability have included long examination times, time-consuming repositioning procedures, and the difficulty of combining the various steps of examination into a single imaging procedure. The development of concomitant data acquisition from several surface coils, new and fast sequences, and moving table platforms have contributed to an improvement in the practicability of whole-body MRI for assessing distant metastasis staging in patients who have malignant tumors (31-33).
Our meta-analysis had several limitations that must also be considered. First, publication bias is one well-described drawback of diagnostic meta-analyses because studies with meaningful results have a higher likelihood of being published than those with nonsignificant results. Furthermore, we also excluded non-original articles (conference abstracts, comments, letters to editors, etc.) that could have resulted in publication bias. However, the publication bias for 18FDG PET-CT and contrast-enhanced MRI groups in our meta-analysis was insignificant (P>0.05). Second, we could did not carry out subgroup analyses based on each primary tumor location as individual patient data would have been needed. The primary tumor locations could influence the organ-sites of distant metastases. 18FDG PET-CT and contrast-enhanced MRI have various advantageous qualities in the detection of malignant lesions in different organs (14,21-25). Therefore, the locations of primary tumors could have an impact on the diagnostic accuracy of 18FDG PET-CT and contrast-enhanced MRI. Third, the qualitative manners for interpreting 18FDG PET-CT and contrast-enhanced MRI were used in most of the included studies, and there could have been a risk of subjective interpretation. Fourth, the histopathologic examination from biopsies for confirmation of distant metastatic lesions was not obtained from all the positive lesions. However, follow-up results from renewed imaging examinations also served as a benchmark in the absence of histologic confirmation. The establishment of a well-accepted reference standard is a challenging aspect of all diagnostic studies which assess different imaging procedures for the identification of distant metastasis.
Conclusions
18FDG PET-CT and contrast-enhanced MRI both have high diagnostic performances in relation to distant metastasis staging in patients with malignant tumors. The subgroup analysis highlights that 18FDG PET-CT and contrast-enhanced MRI may hold different advantages for distant metastasis staging in patients with various types of tumor. Both 18FDG PET-CT and contrast-enhanced MRI may be utilized as first-line imaging techniques for detecting distant metastasis in patients with malignant tumors.
Acknowledgments
Funding: The project is supported by Health Commission of Guangxi Province, China (No. S2018077) and Science and Technology Department of Guangxi Province, China (No.2017GXNSFAA198287).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xu G, Zhao L, He Z. Performance of whole-body PET/CT for the detection of distant malignancies in various cancers: a systematic review and meta-analysis. J Nucl Med 2012;53:1847-54. [Crossref] [PubMed]
- Xu GZ, Li CY, Zhao L, et al. Comparison of FDG whole-body PET/CT and gadolinium-enhanced whole-body MRI for distant malignancies in patients with malignant tumors: a meta-analysis. Ann Oncol 2013;24:96-101. [Crossref] [PubMed]
- Ohno Y, Nishio M, Koyama H, et al. Comparison of the utility of whole-body MRI with and without contrast-enhanced Quick 3D and double RF fat suppression techniques, conventional whole-body MRI, PET/CT and conventional examination for assessment of recurrence in NSCLC patients. Eur J Radiol 2013;82:2018-27. [Crossref] [PubMed]
- Ohno Y, Koyama H, Yoshikawa T, et al. Three-way comparison of whole-body MR, coregistered whole-body FDG PET/MR, and integrated whole-body FDG PET/CT imaging: TNM stage and assessment capability for non-small cell lung cancer patient s. Radiology 2015;275:849-61. [Crossref] [PubMed]
- Ohno Y, Yoshikawa T, Kishida Y, et al. Diagnostic performance of different imaging modalities in the assessment of distant metastasis and local recurrence of tumor in patients with non-small cell lung cancer. J Magn Reson Imaging 2017;46:1707-17. [Crossref] [PubMed]
- Sekine T, Barbosa FG, Sah BR, et al. PET/MR outperforms PET/CT in suspected occult tumors. Clin Nucl Med 2017;42:e88-95. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a general linear mixed model approach. J Clin Epidemiol 2006;59:1331-2. [Crossref] [PubMed]
- Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. [Crossref] [PubMed]
- Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA1994;271:703-7.
- heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol Assess 2005;9:1-113. iii. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Song F, Khan K, Dinnes J, et al. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol 2002;31:88-95. [Crossref] [PubMed]
- Antoch G, Vogt FM, Freudenberg LS, et al. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA 2003;290:3199-206. [Crossref] [PubMed]
- Schmidt GP, Baur-Melnyk A, Herzog P, et al. High-resolution whole-body magnetic resonance image tumor staging with the use of parallel imaging versus dual-modality positron emission tomography-computed tomography: experience on a 32-channel system. Invest Radiol 2005;40:743-53. [Crossref] [PubMed]
- Plathow C, Aschoff P, Lichy MP, et al. Positron emission tomography/computed tomography and whole-body magnetic resonance imaging in staging of advnced non small cell lung cancer--initial results. Invest Radiol 2008;43:290-7. [Crossref] [PubMed]
- Yi CA, Shin KM, Lee KS, et al. Non-small cell lung cancer staging: efficacy comparison of integrated PET/CT versus 3.0-T whole-body MR imaging. Radiology 2008;248:632-42. [Crossref] [PubMed]
- Ohno Y, Koyama H, Onishi Y, et al. Non-small cell lung cancer: whole-body MR examination for M-stage assessment--utility for whole-body diffusion-weighted imaging compared with integrated FDG PET/CT. Radiology 2008;248:643-54. [Crossref] [PubMed]
- Ng SH, Chan SC, Yen TC, et al. Pretreatment evaluation of distant-site status in patients with nasopharyngeal carcinoma: accuracy of whole-body MRI at 3-Tesla and FDG-PET-CT. Eur Radiol 2009;19:2965-76. [Crossref] [PubMed]
- Ng SH, Chan SC, Yen TC, et al. Comprehensive imaging of residual/recurrent nasopharyngeal carcinoma using whole-body MRI at 3 T compared with FDG-PET-CT. Eur Radiol 2010;20:2229-40. [Crossref] [PubMed]
- Chan SC, Wang HM, Yen TC, et al. 18F-FDG PET/CT and 3.0-T whole-body MRI for the detection of distant metastases and second primary tumours in patients with untreated oropharyngeal/hypopharyngeal carcinoma: a comparative study. Eur J Nucl Med Mol Imaging 2011;38:1607-19. [Crossref] [PubMed]
- Ng SH, Chan SC, Yen TC, et al. PET/CT and 3-T whole-body MRI in the detection of malignancy in treated oropharyngeal and hypopharyngeal carcinoma. Eur J Nucl Med Mol Imaging 2011;38:996-1008. [Crossref] [PubMed]
- Duo J, Han X, Zhang L, et al. Comparison of FDG PET/CT and gadolinium-enhanced MRI for the detection of bone metastases in patients with cancer: a meta-analysis. Clin Nucl Med 2013;38:343-8. [Crossref] [PubMed]
- Deng J, Tang J, Shen N. Meta-analysis of diagnosis of liver metastatic cancers: comparison of (18) FDG PET-CT and gadolinium-enhanced MRI. J Med Imaging Radiat Oncol 2014;58:532-7. [Crossref] [PubMed]
- Li Y, Jin G, Su D. Comparison of Gadolinium-enhanced MRI and 18FDG PET/PET-CT for the diagnosis of brain metastases in lung cancer patients: A meta-analysis of 5 prospective studies. Oncotarget 2017;8:35743-9. [PubMed]
- Lee HY, Lee KS, Kim BT, et al. Diagnostic efficacy of PET/CT plus brain MR imaging for detection of extrathoracic metastases in patients with lung adenocarcinoma. J Korean Med Sci 2009;24:1132-8. [Crossref] [PubMed]
- Teefey SA, Hildeboldt CC, Dehdashti F, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology 2003;226:533-42. [Crossref] [PubMed]
- Wudel LJ Jr, Delbeke D, Morris D, et al. The role of [18F] fluorodeoxyglucose positron emission tomography imaging in the evaluation of hepatocellular carcinoma. Am Surg 2003;69:117-24. [PubMed]
- Adams S, Baum R, Rink T, et al. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumors. Eur J Nucl Med 1998;25:79-83. [Crossref] [PubMed]
- Reske SN, Kotzerke J. FDG-PET for clinical use. Results of the 3rd German Interdisciplinary Consensus Conference, Onko-PET III, 21 July and 19 September 2000. Eur J Nucl Med 2001;28:1707-23. [Crossref] [PubMed]
- Barkhausen J, Quick HH, Lauenstein T, et al. Whole-body MR imaging in 30 seconds with real-time true FISP and a continuously rolling table platform: feasibility study. Radiology 2001;220:252-6. [Crossref] [PubMed]
- Goehde SC, Hunold P, Vogt FM, et al. Full-body cardiovascular and tumor MRI for early detection of disease: feasibility and initial experience in 298 subjects. AJR Am J Roentgenol 2005;184:598-611. [Crossref] [PubMed]
- Lauenstein TC, Goehde SC, Herborn CU, et al. Whole-body MR imaging: evaluation of patients for metastases. Radiology 2004;233:139-48. [Crossref] [PubMed]