
Colchicine poisoning complicated by medulla oblongata myelinolysis: a case report
Introduction
Colchicine, a drug used primarily to treat and prevent gout, has a narrow therapeutic index, with no clear distinction between non-toxic and toxic dosages (1). Patients who ingest therapeutic oral doses of colchicine may experience abdominal pain, diarrhea, nausea, and vomiting. These symptoms may lead to hyponatremia, a condition generally defined as a plasma sodium level of <135 mmol/L (2).
The rapid correction of hyponatremia is a common trigger of osmotic demyelination syndrome (ODS) or myelinolysis. This demyelinating condition affects the pontine base, causing central pontine myelinolysis (CPM). The appearance of myelinolysis in other areas of the brain is known as extrapontine myelinolysis (EPM). Both CPM and EPM are associated with rapid osmotic changes (3). To our knowledge; only two cases of medulla oblongata myelinolysis has been reported in literature (4,5). Moreover, to date, this is the first case of colchicine-induced medulla oblongata myelinolysis. We report herein a case of medulla oblongata myelinolysis caused by colchicine poisoning in which the patient’s rapid recovery was not reflected by the brain magnetic resonance imaging (MRI) findings. We present the following case in accordance with the CARE Reporting Checklist.
Case presentation
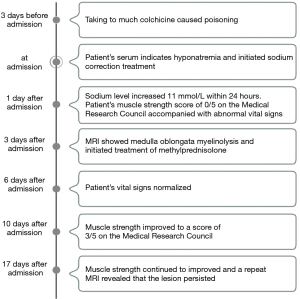
A 34-year-old man presented to the emergency department with nausea, vomiting, diarrhea, and dizziness that arose 3 days after he ingested 15 colchicine pills (total colchicine dose, 0.5 mg). He had a history of gout, and he had felt a terrible pain in his right toe on the day of colchicine ingestion. Based on his previous experience with colchicine, he initially took 3 tablets, which provided no relief; he then took another 3 tablets. The pain eased somewhat but was not eliminated. To further ameliorate the pain, he consumed 9 additional tablets over the next 6 hours. After taking 15 tablets, his pain was substantially eased, but he began to experience dizziness, nausea, and vomiting. These symptoms persisted for 3 days, during which the patient felt increasingly languid. His wife became concerned and brought him to the emergency department. Upon examination, his vital signs and hepatic and renal functions were normal. However, he was very lethargic. His serum sodium concentration of 118 mmol/L indicated hyponatremia. We immediately initiated sodium correction treatment and administered an antiemetic. His sodium level consequently increased to 129 mmol/L within 24 hours (Figure 1).
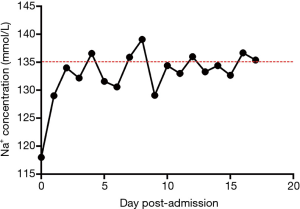
Despite the improved sodium level, he gradually developed weakness in his limbs, which was accompanied by dyspnea, tachycardia, and hypotension. The following vital signs were recorded at that time: respiratory rate, 30 breaths/min; body temperature, 36.7 °C; heart rate, 120 beats/min; and blood pressure, 80/55 mmHg. A blood gas analysis revealed a partial oxygen pressure of 58 mmHg, indicating respiratory failure. We subsequently initiated mechanical ventilation and a dopamine infusion at a rate of 5 mL/h. His pupils had diameters of 3.0 mm. The Babinski sign was intact bilaterally, and his patellar tendon reflexes were active. His serum creatine kinase, aspartate aminotransferase, and lactate dehydrogenase levels and white blood cell counts were normal. However, a Medical Research Council scale evaluation indicated a limb strength score of 0/5, leading to a diagnosis of tetraparesis.
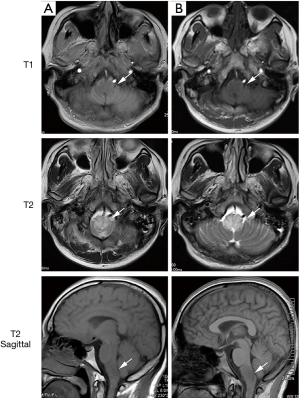
On the third day after admission, brain MRI revealed a lesion in the medulla oblongata that appeared as a hypointense area in T1-weighted images and a hyperintense area in T2-weighted images (Figure 2A). We considered these findings suggestive of medulla oblongata myelinolysis. We therefore administrated intravenous methylprednisolone (dose, 80 mg/d for the first 10 days and 40 mg/d for the next 10 days). After the third day of treatment, the patient’s vital signs normalized. By treatment day 10, his muscle strength had improved to a score of 3/5 on the Medical Research Council scale, and it continued to improve thereafter to a score of 4/5 on day 17. No adverse or unexpected events were observed during treatment. He did not exhibit hepatic or renal insufficiency during this period. Despite these clinical improvements, a follow-up MRI scan revealed that his medulla oblongata lesion persisted (Figure 2B). A summary of the timeline is shown in Figure 3.
Discussion
The excessive administration of colchicine or accidental ingestion of colchicine-containing plants can lead to acute colchicine poisoning, which initially manifests as gastrointestinal effects but may progress to multiorgan dysfunction (6). This latter stage of colchicine poisoning may involve respiratory and circulatory failure and muscle weakness (1,7,8). However, our patient did not present with any clinical manifestations of multiorgan failure or respiratory and circulatory failure upon admission to the hospital. Therefore, we attribute the hyponatremia solely to his initial gastrointestinal reaction to colchicine. The medulla oblongata lesion induced by the rapid correction of hyponatremia then exacerbated the respiratory and circulatory failure and muscle weakening.
Generally, acute flare-ups in adults with gout are treated by administering oral colchicine doses of 1.2 mg/d three to four times per week. The standard oral dose for prophylaxis is 0.5–0.6 mg/d administered three to four times per week. However, even the recommended dose for acute pain can induce gastrointestinal side effects before it provides relief from gout symptoms (9). The initial gastrointestinal symptoms of acute toxicity occur within the first 24 hours, whereas multiorgan failure develops 24–72 hours after ingestion. Additionally, hyponatremia, hypocalcemia, and metabolic acidosis may occur (10). Our patient had no medical history of hyponatremia, had not recently used diuretic agents, and lacked evidence of adrenal, thyroid, pituitary, or renal insufficiency. His medical history implicated colchicine poisoning as the most probable contributor to the development of severe hyponatremia.
As variants of ODS, both CPM and EPM can occur when hyponatremia is not treated properly. EPM generally occurs as a comorbidity of CPM, but some case reports indicate that it can precede CPM or occur in isolation of CPM (3). CPM, the classic presentation, reflects the greater susceptibility of the pontine white matter tracts to this condition, whereas EPM affects other brain regions (e.g., the cerebellum, thalamus, basal ganglia, or subcortical white matter). Although the latter condition is not as rare as previously believed (3,11,12), medulla oblongata myelinolysis remains an extremely rare manifestation of EPM (13). In brain MRI scans, ODS typically appears as hyperintense lesions in the central pons or associated extrapontine structures in T2-weighted and fluid-attenuated inversion recovery images, with corresponding hypointensities in T1-weighted images (11). The findings from our patient’s case were consistent with previously reported observations.
It has been shown that demyelination in patients with ODS can be reversible, with near-complete restoration being possible even in patients whose myelin has entirely disappeared in conventional MRI images (14-17). However, even after the restoration of myelin sheaths, these patients exhibit residual MRI findings that are thought to represent permanent damage (14).
Although the exact pathogenesis of ODS remains unclear, the rapid correction of chronic hyponatremia has been shown to induce pathological changes in animal models (i.e., dogs and rats) (18,19). Currently, most neurologists who treat patients with ODS have reached a consensus that to ensure patient safety, serum sodium levels should not be increased by more than 10 mmol/L/d, although the most recent recommendation suggests a maximum correction rate of 8 mmol/L/d (13). In our patient’s case, the vomiting and diarrhea induced by the ingestion of a high dose of colchicine led to hyponatremia, and the subsequent rapid correction of hyponatremia induced medulla oblongata myelinolysis. We noted that the 11-mmol/L increase in our patient’s serum sodium level on the first day of treatment exceeded the recommended serum sodium correction rate.
Hyponatremia is a relatively common manifestation of colchicine poisoning. Although increasing attention has been given to the diagnosis and treatment of colchicine poisoning and the associated serious complications, this report is, to our knowledge, the first to describe medulla oblongata myelinolysis caused by colchicine poisoning. Our findings from this case have enriched our understanding of the clinical and neuroradiological characteristics of medulla oblongata myelinolysis. Our observations demonstrate the risks associated with rapid sodium correction and proves that MRI findings and clinical symptoms are not always well correlated. In this case, the persistent medulla oblongata lesion in MRI scans was inconsistent with the patient’s good response to methylprednisolone therapy and subsequent clinical recovery. The lack of subsequent dynamic evolution of MRI was a limitation in this study. We attempted to advise the patient to review the MRI, but unfortunately, the patient refused due to long-distance travel.
Conclusions
We report a rare case of medulla oblongata myelinolysis induced by a rapid recovery from colchicine toxicity-induced hyponatremia. This case clarifies the clinical and neuroradiological characteristics of medulla oblongata myelinolysis. Moreover, the patient’s good outcome following methylprednisolone treatment was not accompanied by a resolution of the medulla oblongata lesion, thus confirming that in such cases, MRI findings may not be indicative of the actual clinical situation.
Acknowledgments
Funding: This work was supported by a grant from the National Natural Science Foundation of China to MD (Grant No. 31872772).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-627). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Finkelstein Y, Aks SE, Hutson JR, et al. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol (Phila) 2010;48:407-14. [Crossref] [PubMed]
- Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol 2014;170:G1-47. [Crossref] [PubMed]
- Kumar S, Fowler M, Gonzalez-Toledo E, et al. Central pontine myelinolysis, an update. Neurol Res 2006;28:360-6. [Crossref] [PubMed]
- Kulczycki J, Kozlowski P, Iwinska-Buksowicz B. Central myelinolysis of medulla oblongata. Case report. Neurol Neurochir Pol 1994;28:757-61. [PubMed]
- Bhagavan BS, Wagner JA, Juanteguy J. Central pontine myelinolysis and medullary myelinolysis. Arch Pathol Lab Med 1976;100:246-52. [PubMed]
- Horioka K, Tanaka H, Isozaki S, et al. Acute colchicine poisoning causes endotoxemia via the destruction of intestinal barrier function: the curative effect of endotoxin prevention in a murine model. Dig Dis Sci 2020;65:132-40. [Crossref] [PubMed]
- Kuncl RW, Duncan G, Watson D, et al. Colchicine myopathy and neuropathy. N Engl J Med 1987;316:1562-8. [Crossref] [PubMed]
- Ghosh PS, Emslie-Smith AM, Dimberg EL. Colchicine-induced myoneuropathy mimicking polyradiculoneuropathy. J Clin Neurosci 2014;21:331-2. [Crossref] [PubMed]
- Varughese GI, Varghese AI, Tahrani AA. Colchicine: time to rethink. N Z Med J 2007;120:U2429. [PubMed]
- Güven AG, Bahat E, Akman S, et al. Late diagnosis of severe colchicine intoxication. Pediatrics 2002;109:971-3. [Crossref] [PubMed]
- Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 2014;21:1443-50. [Crossref] [PubMed]
- Zhuang L, Xu Z, Li Y, et al. Extrapontine myelinolysis associated with pituitrin: case report and literature review. BMC Neurol 2014;14:189. [Crossref] [PubMed]
- Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry 2004;75 Suppl 3:iii22-8. [Crossref] [PubMed]
- Yuh WT, Simonson TM, D'Alessandro MP, et al. Temporal changes of MR findings in central pontine myelinolysis. AJNR Am J Neuroradiol 1995;16:975-7. [PubMed]
- Menger H, Jorg J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J Neurol 1999;246:700-5. [Crossref] [PubMed]
- Floris G, Di Stefano F, Melis R, et al. Isolated bipallidal lesions caused by extrapontine myelinolysis. Neurology 2013;81:1722-3. [Crossref] [PubMed]
- Kallakatta RN, Radhakrishnan A, Fayaz RK, et al. Clinical and functional outcome and factors predicting prognosis in osmotic demyelination syndrome (central pontine and/or extrapontine myelinolysis) in 25 patients. J Neurol Neurosurg Psychiatry 2011;82:326-31. [Crossref] [PubMed]
- Laureno R. Central pontine myelinolysis following rapid correction of hyponatremia. Ann Neurol 1983;13:232-42. [Crossref] [PubMed]
- Kleinschmidt-DeMasters BK, Norenberg MD. Rapid correction of hyponatremia causes demyelination: relation to central pontine myelinolysis. Science 1981;211:1068-70. [Crossref] [PubMed]


