
Medically inoperable Merkel cell carcinoma of the head and neck treated with stereotactic body radiation therapy: a case report
Introduction
Merkel cell carcinoma (MCC) is a rare neuroendocrine malignancy that usually occurs in sun-exposed areas of the skin such as the head and neck, shoulders, and upper limbs. A review of the Surveillance, Epidemiology and End Results (SEER) database reported the head and neck region to be the most common location for MCC, of which the majority involved lesions of the face (1). MCC is more likely to develop in elderly patients than in younger patients, with a mean diagnosis age of 76 years for women and 74 years for men (2). Despite having a low age-adjusted incidence rate of 0.18–0.44 cases per 100,000 persons, MCC is a rapidly progressive disease, often with an aggressive primary tumour and a high risk of distant metastases (3). Major risk factors predisposing individuals to MCC include exposure to ultraviolet (UV) radiation, Merkel cell polyomavirus (MCPyV) infection and immunosuppression (4). Conventional treatment methods are currently limited by their toxicity, suitability to patient preferences, and social barriers to accessing care (5). Stereotactic body radiation therapy (SBRT) refers to the use of a small number of image-guided, high dose radiation treatments delivered to a precisely defined target tumour. This case describes the use of SBRT for a patient with locally advanced MCC and significant medical comorbidities who was not deemed to be a surgical candidate. We present the following case in accordance with the CARE Reporting Checklist.
Case presentation
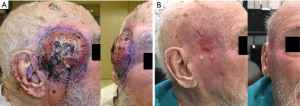
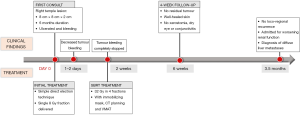
An 88-year-old male presented with a history of a small lesion of 6-month duration in the right temple that began to grow rapidly in the 2 months prior to diagnosis. At first consult, the tumour measured approximately 8-cm wide and 1 to 2-cm thick, involved the right temple and extended to the right side of his face (Figure 1A). The lesion was noted to be ulcerated and bleeding. There was no obvious lymphadenopathy of the neck, right parotid or post auricular region. A biopsy confirmed MCC based on morphology and immunohistochemistry that was positive for synaptophysin and CK20.
The patient’s medical history included diagnoses of insulin dependent type 2 diabetes, congestive heart failure, emphysema, atrial fibrillation requiring bipolar ventricular pacemaker, and renal impairment (creatinine ~250 µmol/L). Additional comorbidities included hypertension, hypothyroidism, benign prostatic hypertrophy, seronegative rheumatoid arthritis, and mild memory impairment.
The patient was assessed in a multidisciplinary cancer clinic by a head and neck surgeon and radiation oncologist. Due to the patient’s extensive medical comorbidities, advanced age and tumour extent, surgery was not recommended. Medical oncology consultation and staging scans were not ordered following discussion with the patient and his family. Radiation was recommended to decrease bleeding and achieve tumour control. In order to start treatment urgently, on the same day as the initial consultation, the tumour was irradiated with a simple direct electron technique delivering a single 8 Gy fraction. Then, an additional 32 Gy in 4 fractions over 2 weeks were then delivered using SBRT with an immobilizing mask, computed tomography (CT) planning and volumetric modulated arc treatment (VMAT).
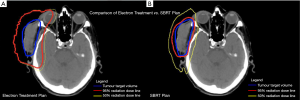
The tumour bleeding decreased within 2–3 days and stopped completely within 1–2 weeks of initiating treatment. Significant tumour shrinkage was noted after three treatments and there was no residual tumour at the 4-week follow-up visit. The skin was well-healed with a small region of erythema and residual desquamation (Figure 1B). The patient did not experience xerostomia, dry eye or conjunctivitis, which are potential side effects associated with conventional radiotherapy to the right side of the face. Figure 2 illustrates the radiation dose plans for electron and SBRT treatments. Three months following the completion of treatment, there was no evidence of loco-regional recurrence, but the patient was admitted to hospital with worsening renal function, and subsequently found to have diffuse liver metastases (Figure 3).
Discussion
The incidence of MCC has risen over the past two decades, with an 8% annual increase between 1986 and 2011 (6), and a current incidence of approximately 0.24 per 100,000 person-years in the United States (7). MCC is the second most common cause of death from non-melanoma skin cancer and the most aggressive cutaneous malignancy with a 30% mortality rate within 5 years of diagnosis (7-10).
Treatment for advanced head and neck MCC presents substantial oncological and reconstructive challenges. Wide local excision (WLE) with or without adjuvant radiotherapy following surgery is the mainstay of treatment during the initial stages of disease (11). Excision margins are dictated by the site of the tumour and typically range from 0.5 to 3.0 cm, although they are often narrower to preserve local structures (11,12). Wide surgical margins in this region can result in substantial functional and cosmetic deformities due to the proximity of important organs. Furthermore, it is difficult to predict the location of nodal metastases given the varied lymphatic drainage in this region (13). The challenges of surgical treatment are compounded by the elderly population in which MCC predominantly presents.
Radiation therapy can be a safe and non-invasive alternative for patients who do not undergo surgery. A review of the current literature suggests that fractionation schedules used to treat MCC vary, with most studies reporting 2–2.5 Gy fractions (14-17). The efficacy of larger doses has yet to be elucidated, although one study reported an in-field tumour control of approximately 80% using a single 8 Gy fraction (14). A summary of findings from retrospective studies examining the efficacy of radiation therapy for MCC is provided in Table 1.

Full table
SBRT is a treatment approach that typically utilizes doses of more than 5 Gy per fraction for a total of 1–5 fractions. Possible advantages include high precision in targeting the tumour and protecting surrounding tissues, and shorter treatment duration while providing similar or better cancer control compared to other techniques (14). For example, the number of treatments may be reduced from 25–30 with standard fractionation to only 4–5 treatments using a SBRT technique. This may be of particular importance for medically frail or elderly patients who have difficulty travelling to and from the cancer centre for daily visits.
The optimal treatment for patients with unresectable/medically inoperable MCC is not well defined. Although the efficacy and safety of SBRT are well established in the treatment of many other disease sites (e.g., lung, liver, bone, brain), there are no prospective data examining the effectiveness of SBRT for treating MCC. This case report illustrates the potential benefits of SBRT in the elderly population, including symptom relief, tumour shrinkage, limited side effects and fewer treatment visits. Clinical research studies are required in this patient population.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-258). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee J, Poon I, Balogh J, et al. A review of radiotherapy for merkel cell carcinoma of the head and neck. J Skin Cancer 2012;2012:563829. [Crossref] [PubMed]
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol 2010;37:20-7. [Crossref] [PubMed]
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol 2008;58:375-81. [Crossref] [PubMed]
- Czapiewski P, Biernat W. Merkel Cell Carcinoma - Recent Advances in the Biology, Diagnostics and Treatment. Int J Biochem Cell Biol 2014;53:536-46. [Crossref] [PubMed]
- Menjak IB, Petrella TM, Lee JW. Curative Stereotactic Ablative Radiotherapy for a Locally Advanced Basal Cell Carcinoma in an Elderly Patient. J Oncol Pract 2018;14:389-91. [Crossref] [PubMed]
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol 2005;89:1-4. [Crossref] [PubMed]
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol 2003;49:832-41. Erratum in: J Am Acad Dermatol 2004;50:733. [Crossref] [PubMed]
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. A clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol 1985;9:95-108. [Crossref] [PubMed]
- Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol 2005;23:2300-9. [Crossref] [PubMed]
- Mott RT, Smoller BR, Morgan MB. Merkel cell carcinoma: a clinicopathologic study with prognostic implications. J Cutan Pathol 2004;31:217-23. [Crossref] [PubMed]
- Hitchcock CL, Bland KI, Laney RG 3rd, et al. Neuroendocrine (Merkel cell) carcinoma of the skin. Its natural history, diagnosis, and treatment. Ann Surg 1988;207:201-7. [Crossref] [PubMed]
- Al-Ghazal SK, Arora DS, Simpson RH, et al. Merkel cell carcinoma of the skin. Br J Plast Surg 1996;49:491-6. [Crossref] [PubMed]
- Gillenwater AM, Hessel AC, Morrison WH, et al. Merkel cell carcinoma of the head and neck: effect of surgical excision and radiation on recurrence and survival. Arch Otolaryngol Head Neck Surg 2001;127:149-54. [Crossref] [PubMed]
- Iyer JG, Parvathaneni U, Gooley T, et al. Single-fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer Med 2015;4:1161-70. [Crossref] [PubMed]
- Sundaresan P, Hruby G, Hamilton A, et al. Definitive radiotherapy or chemoradiotherapy in the treatment of Merkel cell carcinoma. Clin Oncol (R Coll Radiol) 2012;24:e131-6. [Crossref] [PubMed]
- Veness M, Foote M, Gebski V, et al. The role of radiotherapy alone in patients with merkel cell carcinoma: reporting the Australian experience of 43 patients. Int J Radiat Oncol Biol Phys 2010;78:703-9. [Crossref] [PubMed]
- Harrington C, Kwan W. Outcomes of Merkel cell carcinoma treated with radiotherapy without radical surgical excision. Ann Surg Oncol 2014;21:3401-5. [Crossref] [PubMed]


