
Regulation of lncRNA-H19/miR-140-5p in cartilage matrix degradation and calcification in osteoarthritis
Introduction
Osteoarthritis (OA) is a chronic degenerative joint disease, whose incidence may increase with age and more common in the elderly. OA is defined by insufficient cartilage matrix synthesis, degeneration of the articular cartilage, development of an osteophyte, and synovitis. The pathogenesis of OA involves various physiological and pathological processes of the body, including articular cartilage anabolic dysfunction, catabolism, and degradation of the extracellular matrix (1). However, the exact mechanism of OA is unclear.
In OA, the epigenetic modifications of long non-coding RNA (lncRNA) and microRNA (miRNA) have been reported to play a vital part (2,3). For example, HOTTIP expression is up-regulated, while homeobox gene A13 expression is down-regulated in OA cartilage tissue, which inhibits integrin α1 synthesis and causes cartilage destruction (4). Down-regulation of lncRNA MEG3 in OA cartilage tissue leads to OA progression via regulating the miR-16/SMAD7 axis (5). Like lncRNA, expression changes in various miRNAs can also affect OA. For example, the up-regulation of miR-146a and miR-98 can promote the progress of OA (3), and miR-16-5p also participates in the occurrence and development of OA (5). In addition, exosomes carrying miR-140-5p are reported to promote the proliferation and migration of rat chondrocytes in OA without disrupting the secretion of extracellular matrixes (6).
Studies have shown that the expression of lncRNA H19 increased in OA cartilage tissue to regulate miR-675 and affect synthesis and catabolism of cartilage, which speculates that H19 is a potential target for stimulating cartilage recovery (7). However, the effects of expression changes of H19 on the degradation and calcification of the OA cartilage matrix are unclear. In this report, it was indicated that H19 could target miR-140-5p to inhibit its expression, and the regulatory axis H19/miR-140-5p had a potential role in regulating degradation and calcification of the matrix of the OA cartilage. This study may supply a potential target and basis for the diagnosis and treatment of OA chondrocyte degeneration.
Methods
Clinical data
Cartilage tissue samples from 20 patients with primary knee OA who underwent joint replacement. Samples of knee cartilage tissue from 20 post-traumatic amputation patients were obtained in our hospital's orthopedic department from January 2015 to June 2019. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All specimens were approved by our ethics committee and get the informed consent of the patients. Among the twenty patients with primary knee OA, 13 males and seven females were included, with an average age of 52.6 years old (ranging from 39 to 72 years). There were 18 cases of traumatic amputation, including 12 males and six females, with an average age of 41.8 years old (ranging from 29 to 61 years).
Reagents and consumables
TRIzol kit was bought from Invitrogen (USA). PrimeScript TM RT Master Mix and LipofecamineTM2000 kits were obtained from Eurofins MWG Operon (Germany). Human chondrocyte cell lines (HC-A) were purchased from Shanghai Aolu Biotechnology Co., Ltd. SYBR qPCR Mix and cell counting kit 8 (CCK-8) was purchased from TaKaRa (Japan), and the phosphatase detection kit was purchased from China Jiancheng Biological Engineering Company. The bicinchoninic acid (BCA) kit was bought from Thermo Fisher Scientific (USA). Polyvinylidene fluoride (PVDF) membrane was bought from Millipore (USA). The primary antibodies against MMP-1, MMP-13, type II collagen α1 (COL2A1), GAPDH, and relative secondary antibodies were all bought from Abcam (USA).
Total RNA extraction and real-time quantitative polymerase chain reaction (RT-qPCR)
The TRIzol kit was used to extract complete RNA from cartilage tissue, relative healthy tissues, and chondrocytes, and a UV spectrophotometer was used to measure RNA concentrations. Complementary DNA (cDNA) was synthesized according to the instructions of the PrimeScript TM RT Master Mix kit, under the following reaction conditions: 40 °C for 6 min, 65 °C for 25 min. Finally, we got the cDNA product to store at −80 °C for a further experiment in qPCR. The reaction system of RT-qPCR was at a total volume of 20 µL, including 2 µL cDNA product, 0.4 µL 50× ROX, 10 µL SYBR qPCR Mix, 0.8 µL upstream primer and 0.8 µL downstream primer and RNase water supplemented to 20 µL. The reaction conditions were as follows that first denaturation at 95 °C for 1 min, then 95 °C for 30 s and 60 °C for 40 s, for a total of 40 cycles. Each experiment was performed in 3 replicates, and each sample was repeated for three times. The relative expression of a gene was expressed using 2–ΔΔCt using a relative quantitative method. All steps for this experiment were performed on ice. Primer sequences were shown in Table 1.
Full table
Cell culture
HC-A cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 medium, having 10% fetal bovine serum. All the cells were cultured at 37 °C in a humid sterile incubator holding 5% CO2, with the medium changed once every two days.
Cell transfection
The cells were seeded into plates with 6 wells. After 24 h, when cell density reached 70%, cells were divided into six treatment groups including H19 interference group (si-H19), negative transfection group (si-Control), miR-140-5p mimic group (mimic), mimic negative control group (mimic-NC) and miR-140-5p inhibitor group (inhibitor) and negative control group (inhibitor-NC) inhibitor group. For transfection, mix LipofecamineTM2000 with si-H19, si-Control, mimic, mimic-NC, inhibitor, inhibitor-NC, inhibitor + si-H19, inhibitor-NC + si-H19 separately to make the final concentration of 100 nmol/L firstly, add the mixture to the cells and incubate for 6 h, then change to the complete medium and culture for 24 h. Finally, cells were collected for later experiments.
Cell proliferation assay
After transfection of si-H19 or si-Control, cells were collected and seeded into 96-well plates at a density of 1,000 cells/well, which were cultured for 0, 24, 48, or 72 h. The CCK-8 kit solution was added to each well at a final concentration of 10% and incubated for 1 h in the dark. Finally. The absorbance (OD) values were detected using a microplate reader at the wavelength of 450 nm.
Analysis of apoptosis by flow cytometry
After transfection with si-H19 and si-Control, cells in the logarithmic growth phase were collected and digested, which were next washed twice with cold PBS and resuspend using 1 mL of the combined buffer to make the cell density of 1×106/mL. 100 µL of cell suspension was added to a 5 mL flow tube, followed by 10 µL Annexin-V-FITC and 10 µL propidium iodide (PI) for staining. Subsequently, it was incubated at room temperature for 15 minutes, avoiding the light. Finally, the apoptotic rate was analyzed by flow cytometry. All determinations were performed in three independent experiments.
Western blotting
The total protein of the transfected cells was extracted, then the protein concentration was determined by the BCA kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA). After denatured, 20 µg of total protein was separated by running sodium lauryl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, United States). After being blocked in 5% skim milk, the membrane was incubated with primary antibodies against MMP-1, MMP-13, COL2A1, and GAPDH overnight at 4 °C. Then the membrane was washed with Tris-buffered saline containing Tween 20 (TBST), which was then incubated with the secondary antibody for 2 h at room temperature and washed with TBST. At last, signals of protein bands could be detected after reacting with enhanced chemiluminescence (ECL) western blot detection reagents. The gray value of the bands was analyzed using ImageJ software, with GAPDH as a loading control.
MMPs activity detection
The experiments were strictly carried out according to the instructions pro-MMP-1 and MMP-13 ELISA kits (R&D, USA). The sensitivity of the kit was 0.02 and 0.009 ng/mL, respectively.
Measurement of alkaline phosphatase (ALP)
ALP activity was detected using an ALP detection kit. Cells from each transfection group were washed with PBS and lysed with a solution containing 20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% Triton X-100, 0.02% NaN3, and 1 Triton aprotinin. The lysate was homogenized using a pipette, then incubated at 37 °C for 15 min. The ALP activity was measured using a spectrophotometer.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 software, with all data being expressed as mean ± standard deviation. Comparisons between groups were performed by t-test or one-way ANOVA, with P<0.05 considered statistically significant, where one-way ANOVA was performed for post hoc test.
Results
Expression and function of H19 in OA
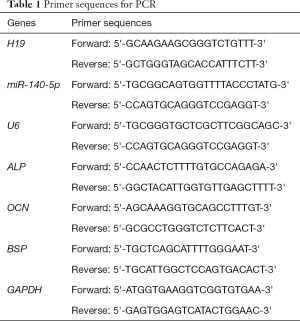
Compared with healthy tissues, the expression of H19 in OA cartilage tissue increased significantly (P<0.05, Figure 1A). Transient transfection of si-H19 into could inhibit the expression of H19 significantly (P<0.05, Figure 1B), which could promote cell proliferation significantly and maintained for at least 72 h due to the silence of H19 (P<0.05, Figure 1C). The result of flow cytometry showed that silencing H19 by transfection of si-H19 could inhibit apoptosis significantly (P<0.05) (Figure 1D). Also, si-H19 in HC-A cells could lead to low expression of Bax and high expression of Bcl-2 (Figure 1E), with the difference statistically significant (P<0.05).
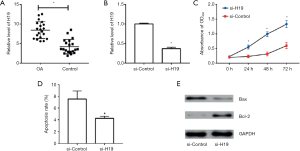
Effect of H19 on extracellular matrix-related genes
The expression of MMP-1 and MMP-13 was suppressed by transient transfection of si-H19 in HC-A cells, while the expression of COL2A1 increased, with the difference statistically significant (both P<0.05, Figure 2A,B).
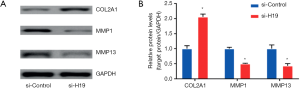
Effect of H19 on related indexes of chondrocyte ossification
After the transfection of si-H19, mRNA levels of ALP, OCN, and BSP were down-regulated significantly (all P<0.05), as shown in Figure 3A. Moreover, si-H19 transfection could also inhibit the ALP activity of chondrocytes, with statistical significance (P<0.05, Figure 3B).
miR-140-5p is negatively regulated by H19
Compared with healthy tissues, the expression of miR-140-5p was decreased in OA cartilage tissues significantly (P<0.05, Figure 4A). It was shown that the expression of H19 and miR-140-5p was negatively correlated (r=–0.98, P<0.001, Figure 4B). The expression of miR-140-5p could also be upregulated by si-H19 significantly (P<0.05, Figure 4C). The expression of miR-140-5p could be increased by transfection of miR-140-5p mimic, while it could be inhibited by transfection of miR-140-5p inhibitor (Figure 4C).
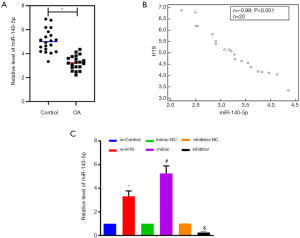
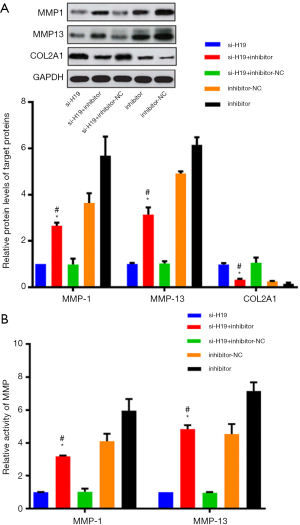
Effect of H19/miR-140-5p on extracellular matrix-related genes
Chondrocytes were simultaneously transfected with miR-140-5p inhibitor and si-H19. Compared with the si-H19 group, the expression level and activity of MMP-1 and MMP-13 were both up-regulated, while the expression level of COL2A1 was down-regulated, where both the differences were statistically significant (P<0.05, Figure 5A,B). Also, compared with the si-H19 + inhibitor-NC group, the expression level and activity of MMP-1 and MMP-13 were both up-regulated, while the expression of COL2A1 was down-regulated in the si-H19 + miR-140-5p inhibitor group, where both the differences were statistically significant (P<0.05, Figure 5A,B).
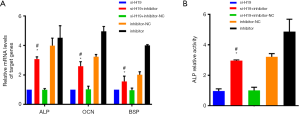
Effect of H19/miR-140-5p on related indexes of chondrocyte ossification
After transfecting with miR-140-5p inhibitor and si-H19 simultaneously, the mRNA level of ALP, OCN and BSP were up-regulated compared with the si-H19 group alone (all P<0.05, Figure 6A), while the ALP activity of chondrocytes was inhibited significantly (P<0.05, Figure 6B). Compared with the si-H19 + inhibitor-NC group, mRNA levels of ALP, OCN, and BSP in the miR-140-5p inhibitor + si-H19 group were up-regulated significantly (all P<0.05, Figure 6A), while the ALP activity of chondrocytes was inhibited, with the difference statistically significant (all P<0.05, Figure 6B).
Discussion
In this study, the results indicated that high expression of H19 might participate in the development of OA by regulating the process of chondrocyte proliferation, apoptosis, matrix degradation and calcification, which was also closely related to low expression of miR-140-5p. In addition, negative regulation of miR-140-5p was shown to be one of the essential mechanisms by which H19 was able to regulate degradation and calcification of the OA chondrocyte matrix.
OA, a common joint degenerative disease in the elderly, is characterized by the degradation of cartilage structure and function, with most of the lesions being located in the tissue around the joints, which may eventually lead to joint pain and limited functional activity (8). However, the treatment results and prognosis of OA are not satisfactory. For example, the most used non-steroidal anti-inflammatory and analgesics may only improve the quality of life by alleviating pain and inflammation. And the intra-articular injection of hormones or hyaluronic acid is only suitable for relieving OA inflammation symptoms (9-11). Therefore, in-depth researches about the pathogenesis of OA may supply a detailed theoretical basis for the treatment of this disease.
The MMP family plays a vital role in the pathological process of OA. It is reported that the family of MMP proteases is widely distributed in the human body, with the primary function of controlling the degradation of extracellular matrix components, considered as one of the primary factors in the physiological reconstruction and pathological damage processes (12,13). In OA, MMP is expressed in chondrocytes, synovial cells, and osteoclasts, which is intimately involved in the hydrolysis of cartilage matrix macromolecules, including type II collagen and polysaccharides, and ultimately destroys the structural and functional integrity of the extracellular matrix of articular cartilage. As a kind of collagenase, MMP-1 is one of the rate-limiting enzymes and the most typical type II collagen in the degradation of cartilage matrix, which can degrade collagen in the triple helix region of collagen fibers and induce the activation of other MMP family members to play an essential role in the development of OA (14). MMP-13, also known as collagenase 3, is a newly discovered member of the MMP family and expressed in connective tissue. In healthy cartilage tissues, there is little MMP-13 expressed or no expression. Only during the process of cartilage osteogenesis, MMP-13 secretion can degrade the cartilage matrix for osteogenesis. At the same time, it is reported that MMP-13 is a potent protease, which has a particular degradation effect on collagen and non-collagen substances in the extracellular matrix, especially on the type II collagen (15). Therefore, MMP-13 is the most effective protease for cleavage of U-shaped collagen. Recent studies have confirmed that MMP-13 is not only overexpressed in damaged cartilage but also upregulated in synovial tissue, calcified cartilage, and subchondral bone (16). In this study, the expression of MMP-1 and MMP-13 in chondrocytes was down-regulated by overexpression of miR-140-5p or silencing H19, suggesting that H19 might be involved in the development of OA by regulating the extracellular matrix.
Also, in order to confirm the effect of H19 and miR-140-5p on the calcification of chondrocytes furtherly, we detected the expression levels of ALP, OCN, BSP and the activity of ALP in chondrocytes, finding that expression levels of OCN and BSP were inhibited by H19 silence significantly, as well as the inhibition of ALP activity. Several studies have confirmed that H19 can promote osteogenic differentiation of stem cells and up-regulate the expression of OCN and ALP in bone marrow mesenchymal stem cells (17), suggesting that inhibition of H19 expression may inhibit ossification of chondrocytes. Our further research showed that inhibition of miR-140-5p could partially reverse the downregulation of ALP activity, OCN, and BSP expression levels caused by H19 silence. And the promotion effect on osteogenic differentiation of mesenchymal stem cells by inhibition of miR-140-5p was confirmed in the published data (18,19). The result indicated that H19 negatively regulates miR-140-5p in chondrocytes so that miR-140-5p silence could rescue the decreased levels of osteogenic markers (ALP, OCN, and BSP) caused by H19 inhibition.
In summary, the results showed that the expression of H19 in OA cartilage tissues was up-regulated, which may promote the degradation and ossification of the extracellular chondrocyte matrix by inhibiting miR-140-5p, thus participating in OA progression.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-929
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-929). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All specimens were approved by our ethics committee and get the informed consent of the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DeRogatis M, Anis HK, Sodhi N, et al. Non-operative treatment options for knee osteoarthritis. Ann Transl Med 2019;7:S245. [Crossref] [PubMed]
- Abbasifard M, Kamiab Z, Sadeghi I, et al. The role and function of long non-coding RNAs in osteoarthritis. Exp Mol Pathol 2020.104407. [Crossref] [PubMed]
- Kourtis A, Adamopoulos PG, Papalois A, et al. Quantitative analysis and study of the mRNA expression levels of apoptotic genes BCL2, BAX and BCL2L12 in the articular cartilage of an animal model of osteoarthritis. Ann Transl Med 2018;6:243. [Crossref] [PubMed]
- Mao G, Kang Y, Lin R, et al. Long Non-coding RNA HOTTIP Promotes CCL3 Expression and Induces Cartilage Degradation by Sponging miR-455-3p. Front Cell Dev Biol 2019;7:161. [Crossref] [PubMed]
- Xu J, Xu Y. The lncRNA MEG3 downregulation leads to osteoarthritis progression via miR-16/SMAD7 axis. Cell Bioscience 2017;7:69. [Crossref] [PubMed]
- Tao SC, Yuan T, Zhang YL, et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017;7:180. [Crossref] [PubMed]
- Steck E, Boeuf S, Gabler J, et al. Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J Mol Med 2012;90:1185-95. [Crossref] [PubMed]
- Schmelz M, Mantyh P, Malfait AM, et al. Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: mechanism of action in the context of efficacy and safety. Pain 2019;160:2210. [Crossref] [PubMed]
- Huang R, Li X, Xu S, et al. Acupoint injection treatment for primary osteoporosis: a systematic review and meta-analysis of randomized controlled trials. Ann Palliat Med 2019;8:586-95. [Crossref] [PubMed]
- Huang C, Wu Z, Jiang Q. Effect of Qufeng Zhitong capsule on score of pain and knee function in patients with knee osteoarthritis. Chinese Journal of New Drugs 2019.11.
- Huang K, Wu Z, Zhang Z, et al. Hyaluronic acid,p latelet rich plasma and the combination of both in the treatment of osteoarthritis of the knee. Chinese Journal of Osteoporosis 2019;25:1707-11.
- Yu Y, Wang C, Liu J, et al. Evolvement of reconstruction rules about extracellular matrix in fibrogenesis after myocardial infarction. Chinese Heart Journal 2019;31:83-8.
- Wang H, Ding X, Yang L, et al. Differences in the expression of related degrading enzymes in joint fluids of different stages of osteoarthritis. Journal of Clinical Rehabilitative Tissue Engineering Research 2019;23:3609.
- Alamgeer, Hasan UH, Uttra AM, et al. Phytochemicals targeting matrix metalloproteinases regulating tissue degradation in inflammation and rheumatoid arthritis. Phytomedicine 2020:153134.
- Fan K, Jing WU, Qin LI, et al. Advances in matrix metalloproteinase 13 in cartilage remodeling and arthritis. Chinese Pharmacological Bulletin 2018;34:607-11.
- Dai Y, Lu J, Li F, et al. Changes in cartilage and subchondral bone in a growing rabbit experimental model of developmental trochlear dysplasia of the knee. Connective Tissue Research 2019.1-14. [Crossref] [PubMed]
- Zhou P, Li Y, Di R, et al. H19 and Foxc2 synergistically promotes osteogenic differentiation of BMSCs via Wnt‐β‐catenin pathway. J Cell Physiol 2019;234:13799-806. [Crossref] [PubMed]
- Hwang S, Park SK, Lee HY, et al. miR‐140‐5p suppresses BMP2‐mediated osteogenesis in undifferentiated human mesenchymal stem cells. FEBS Lett 2014;588:2957-63. [Crossref] [PubMed]
- Li Z, Jin C, Chen S, et al. Long non-coding RNA MEG3 inhibits adipogenesis and promotes osteogenesis of human adipose-derived mesenchymal stem cells via miR-140-5p. Mol Cell Biochem 2017;433:51-60. [Crossref] [PubMed]


