
The quest for diagnostic approaches of cardiac involvement in polymyositis and dermatomyositis
Introduction
Polymyositis (PM) and dermatomyositis (DM) are rare acquired autoimmune diseases characterized by an inflammatory infiltrate of the skeletal muscles. The incidence of PM/DM is reported to be 2 to 10 cases per million persons (1). Patients with PM/DM usually present with subacute weakness accompanied by elevated creatine kinase (2,3). Skeletal muscle is the main target, but PM and DM are classified as systemic inflammatory autoimmune diseases because of the presence of extra-muscular features. Lung, joints, heart and gastrointestinal tract can be affected (4). Although imperfect and often debated, the classification criteria proposed by Bohan and Peter are still used, including five items: symmetric proximal muscle weakness, elevated serum muscle enzymes, myopathic changes in electromyography, a muscle biopsy suggestive of myositis and the characteristic DM rash (5-8). Muscle biopsy is necessary for definite diagnosis and differential diagnosis. The central pathological finding is diffuse skeletal muscular inflammation. In DM, perivascular infiltration is predominated by CD4+ T cells. In PM, the inflammatory infiltrate is basically composed of endomysial infiltrates of CD8+ T cells and macrophages (9). The most frequent histopathologic finding of cardiac tissue in PM/DM patients with heart disease is myocardial fibrosis. Mononuclear inflammatory infiltrates similar to those in skeletal muscle could also be observed (10). To lower the falsely negative rate, magnetic resonance is a novel tool that can detect muscle edema at early stage and help select the potential area of biopsy (11). PM/DM patients usually respond well to immunotherapy. After the treatment strategy of corticosteroids in combination with immunosuppressive started to be widely used, the prognoses significantly improved (12). The 5-year survival rate has reached 95% in long-term follow-up (13,14). However, cardiac involvement remains a poor prognostic factor despite the overall improvement in survival rate and was the leading cause of death in several studies (14,15).
Thus, timely and accurate diagnosis of cardiac involvement in PM/DM is warranted, but recognition of this disease entity is often delayed in clinical practice. Because cardiovascular involvement is frequent in PM/DM but typically remains subclinical, basic diagnostic considerations, including clinical history, physical examination, common laboratory testing and electrocardiography (ECG), do not always provide enough information. Now that the onset of cardiac involvement is important but often insidious, how can cardiac involvement be identified?
No certain criteria for cardiac involvement in PM/DM has been proposed so far. The diagnostic difficulties have led to the increasing importance of different imaging modalities in the assessment of cardiac involvement in PM/DM. Growing availability and maturity of multi-imaging techniques may facilitate diagnosis with better sensitivity and specificity and improve endomyocardial biopsy by decreasing sampling error. Detection of new cardiac-specific biomarkers and correlation between myocardial involvement and traditional autoantibodies are under investigation. The aim of this review is to illustrate the characteristics of currently available diagnostic approaches for cardiac involvement in PM and DM. Their advantages and limitations will be stated and supported by existing data, and future prospects will be envisaged. We will briefly discuss the possible mechanisms and therapies of cardiac involvement. Hopefully, this review could arouse the awareness of the importance of cardiac involvement in PM/DM, and facilitate the diagnostic process.
Cardiac involvement in PM/DM
PM/DM can be complicated by disorders of extramuscular organs such as the joints, gastrointestinal tract, skin and lung (16). Cardiac involvement is often overlooked in patients with PM/DM. In a recent meta-analysis, the incidence of cardiac involvement in patients with PM/DM ranged between 9% and 72% due to different definitions of cardiac involvement (17). It has been suggested that cardiac involvement in PM/DM should be interpreted as the functional or structural abnormality of heart caused by the sustained inflammatory process of PM or DM in patients with a definite diagnosis of PM/DM, without previous cardiac diseases (for example, congenital heart disease, rheumatic heart disease, ischaemic heart disease and so forth). Different cardiac structures can be affected: heart valves, conduction system, myocardium, endocardium, pericardium, pulmonary and coronary arteries. Accordingly, different cardiovascular diseases can occur: valvular disease, arrhythmias, myocarditis, pericarditis, pulmonary hypertension and coronary heart disease (12,17-21).
The onset of cardiac involvement is often insidious, leading to delayed recognition and intervention (12). Heart failure, myocardial infarction and arrhythmias were considered to be the three major causes of cardiac mortality in patients with PM/DM (18). The reported mortality due to cardiac involvement in adult-onset PM/DM differed among studies. In a study of 160 consecutive patients with a median follow-up of 4.6 years, 27 patients died. Cardiac involvement (4 acute myocardial infarction and 2 congestive heart failure) was the leading cause (15). Similarly, cardiac and pulmonary complications were the most frequent causes of death in the 18 disease-specific deaths that occurred in a longitudinal study of 162 cases (14). Eleven of 20 (55%) of the deaths were caused by a cardiac aetiology in a Hungarian longitudinal study, whereas 12/87 (14%) deaths were due to cardiac causes in a population-based Norwegian study (17).
Mechanisms
Although the events triggering the disease process are poorly understood, recent studies have improved our understanding of the involvement of cardiac tissue in patients with PM/DM (Figure 1). First, traditional cardiovascular risk factors are more prevalent in PM/DM compared with healthy controls (22-25). Diederichsen and colleagues reported that hypertension and diabetes mellitus were more frequent in PM/DM patients (71% vs. 42%; P=0.002, and 13% vs. 0%; P=0.007), and PM/DM patients had higher levels of triglycerides (P=0.0009). Second, systemic and local inflammation exist sustainably in PM/DM patients to different extents. They may have a direct impact or make the heart more susceptible to traditional risk factors (18,26). Third, long-term glucocorticoid treatment may play a role in the deterioration of cardiac involvement, as indicated by the increased prevalence of hypertension and dyslipidaemia after glucocorticoid usage (27,28).
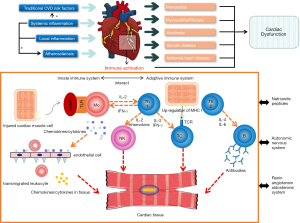
Activation of both the adaptive immune system and innate immune system is involved in the inflammatory process in the muscle tissue, leading to cardiomyopathy in PM/DM. PM and DM are characterized by antibody-mediated and cell-mediated immunological responses. Different cytokines and adhesion molecules with elevated levels in muscle tissue have been reported, such as type-I interferon (IFN), tumour necrosis factor-alpha (TNF-α), interleukin-2 (IL-2), interleukin-1α (IL-1α), intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), in both capillaries and muscle fibres (1). However, it is not known what triggers the upregulation of inflammatory cytokines and adhesion molecules. Kim et al. found that higher VCAM-1 protein expression in muscle and the vasculature in juvenile DM patients might be augmented by TNF-α and regulated by microRNA-126, providing a new pathogenetic mechanism and possible avenue of therapeutic intervention (29). Cytokines and chemokines relevant to cardiomyopathy have not been fully investigated. It has been proposed that the renin-angiotensin-aldosterone system, autonomic system, natriuretic peptides and extracellular matrix may influence innate immunity in a way that leads to heart failure in cardiomyopathy (30). Apart from the damaged muscle fibers, derangement of the coronary microcirculation also contributes greatly to the development of cardiomyopathy (31). Early autoantibody-mediated activation of endothelial cells followed by complement activation could lead to endotheliopathy and take part in the pathogenesis of muscle damage (32). Upregulated inflammatory cytokines similar to the muscle tissue have been detected in endothelium of damaged vessels (33). The dysfunction of macrovascular coronary artery may also worsen the cardiomyopathy, but so far it remains inconclusive (19,34).
Animal models suitable for investigating cardiac involvement in PM or DM have not been reported so far. Nagaraju et al. realized controllable upregulation of major histocompatibility complex (MHC) class I using doxycycline and discovered that H+ T+ mice can develop clinical, biochemical, histological, and immunological features similar to human myositis. Autoantibodies against histidyl-tRNA synthetase were also detected (35). Similar to a previous human study, this study concluded that upregulation of class I MHC expression, an apparently nonspecific event, leads to the specific autoimmune pattern of PM/DM (35,36). However, the disease developed mainly in skeletal muscles rather than cardiac muscles in H+ T+ mice (35). Wang et al. generated MRL-Pdcd1−/− mice by backcrossing C57BL/6-Pdcd1−/− mice with MRL-Faslpl/lpl mice. This model revealed the heart-specific autoimmune predisposition, with 96% of them developing myocarditis between 4 and 8 weeks, which was likely due to the tissue-specific loss of immunological tolerance. High-titre auto-antibodies against cardiac myosin also existed (37,38). It is hoped that a similar pattern could be introduced in myositic mice for researching cardiac involvement in the future. In clinical scenarios, anti-programmed death-1 (PD-1) antibodies can also cause myocarditis. This drug-induced toxicity may provide valuable insight into the occurrence of idiopathic myocarditis in the future (39).
Clinical presentations of cardiac involvement in PM/DM
The occult nature of PM and DM sets barriers in the early diagnosis of myocardial involvement that often develops slowly (40). The most commonly detected clinical heart feature is congestive heart failure, but clinically overt cardiac problems are infrequent (17,19). In the study of Hochberg and colleagues, 37% of the study population had cardiac involvement using Medsger and Masi’s criteria, but only in 3% of them was it clinically evident (41). Bohan and colleagues investigated 153 PM/DM patients, finding nonspecific ST-T abnormalities as the most frequent manifestation, while only 5 patients had congestive heart failure (42). Evident symptoms reminiscent of cardiac involvement are seldom seen in clinical practice.
PM and DM are systemic diseases with different constellations of clinical manifestations and co-existence of features of different extramuscular organ involvement (5,6). Physicians may get confused when judging the causes of certain symptoms. For instance, dyspnoea, the most prevalent symptom in PM/DM patients with myocardial involvement (10,17,43), can be caused by respiratory muscle or lung involvement (20). Careful evaluation is needed to avoid misinterpretation.
Heart disease may appear at any phase of the idiopathic inflammatory myopathies (IIMs) (44). Cardiac manifestations may be present at the initial diagnosis of myositis, developing after the treatment has begun, even when the disease is in remission (20,21,34). Coley and colleagues proposed adenosine monophosphate deaminase (AMP) deficiency, a non-immune mechanism, as a possible explanation for the poor correlation between the suppression of inflammation and a recovery of disease progression (45).
Serum biomarkers
Cardiac troponin (cTn) is composed of three subunits, T, I, and C. Unlike troponin C (cTnC), for which skeletal muscle and cardiac muscle share identical amino acid sequences, both troponin T (cTnT) and I (cTnI) were thought ideally suited for the detection of myocardial damage in most scenarios, as they are expressed as cardio-specific isoforms (46). However, for cTnT, this principle seems not to apply to PM/DM patients. cTnT has been reported to have a closer correlation with serum creatase level than myocardial involvement (47,48). It has been demonstrated in skeletal muscle biopsies from patients with PM that the regenerating skeletal muscle tissue undergoes a phenotype switch, in which previously repressed cTnT is re-expressed (49). Although cTnT is also expressed by skeletal muscle in PM/DM patients, cTnI remains specific to the myocardium (50). Additionally, as a quantitative index, the dynamic changes of cTnI could help monitor the disease progress and therapeutic response. Thus, it is reasonable that we use the cTnI assay for the detection of myocardial involvement with minor cell damage in preference to high-sensitivity assays (51). If cTnI is elevated, the patient is very likely to have myocardial involvement. If cTnI is normal, other cardiac involvement manifestations such as conduction disturbances and structural alternations must be carefully ruled out because troponin leaks out of the cell only after membrane disruption (52).
The main cause of cTnI elevation is considered non-viral myocarditis leading to cardiomyocyte damage via inflammatory activity (51). Coronary heart disease can also result in myocardial injury in PM/DM patients. A meta-analysis including 13,201 patients with IIMs demonstrated a significantly increased coronary artery disease (CAD) risk among IIMs patients, with a pooled risk ratio of 2.24 (95% confidence interval: 1.02–4.92) (53). Rai and colleagues reached a similar conclusion in new cases of inflammatory myopathies in British Columbia (54). Myocardial injury may also develop after tachycardia because of infiltration of the autonomic nervous system and the subsequent shorting of diastole with subendocardial ischaemia (19,46,52). Pulmonary hypertension can also contribute to cTnI elevation (55,56).
To date, various myositis-specific antibodies (MSA) and myositis-associated antibodies (MAA) have been described. Myositis-specific antibodies have a strong association with certain clinical diseases and specific features (57). For example, anti-synthetase antibodies are associated with a clinical syndrome made up of a constellation of clinical features (58). Anti-MDA5 antibodies indicate the presence of interstitial lung disease in patients with PM/DM (59). MAAs are found in inflammatory myopathies and other autoimmune diseases (60). As for cardiac involvement, however, no MSA/MAA has been shown to have a close correlation (61). Love and colleagues reported that a patient group with a positive anti-signal recognition particle (SRP) autoantibody was distinguished by increased palpitations (62). In contrast, Kao and colleagues did not observe overt cardiac involvement in SRP-positive PM patients (63). Recently, Albayda and colleagues found that anti-mitochondrial antibodies were related to a distinct inflammatory myopathy phenotype frequently associated with severe cardiac involvement (71%), including myocarditis, arrhythmia and cardiomyopathy. However, they identified only 7 patients, and the sample size was rather small (64).
ECG
ECG is the basic method to detect rhythm disturbances and conduction alternations in the assessment of PM/DM (44). The reported frequency of ECG abnormalities ranged from 32.5% to 85% (19,31,34,41,42,65-71). ECGs were available in 538 patients with PM or DM in published literature, and most ECG alternations were non-specific. Among them, the three most frequent ECG abnormalities were ST-T abnormalities (17.8%), ventricular premature complexes (6.5%) and bundle branch block (5.6%).
Of 16 autopsied PM/DM patients, 10 had ECG abnormalities, but only 1 of them had pathologic conduction system alternations directly attributable to PM/DM (18). ECG abnormalities, especially conduction defects, occurred frequently, in 77 patients. However, their occurrence and progression did not relate to the severity, activity, or treatment of PM (34). Comparing ECG findings in PM/DM patients with matched healthy controls, a prolonged corrected QT interval was the only difference in two cross-sectional studies (71,72). Therefore, ECG abnormalities occur at a high frequency in PM/DM but lack specificity.
Multimodality imaging: progress in cardiac imaging
Echo
Echo is a widely used and cost effective examination for detecting cardiac functional and structural abnormalities in PM/DM patients in routine clinical practice (10). The most common finding is diastolic dysfunction (43,68). The deterioration of diastolic dysfunction may develop during the disease course due to the ongoing fibrotic transformation of the myocardium, finally leading to clinically symptomatic heart failure. Two other common Echo abnormalities are hyperdynamic left ventricular contraction and prolapse of the mitral valve (43,68). Without evidence of known causes of hyperdynamic function such as fever, hyperthyroidism or anaemia, it is presumed that hyperdynamic left ventricular contraction results from increased arteriovenous shunting across involved skeletal muscle beds and a high output state secondary to increased metabolic demand or released vasoactive amines from diseased skeletal muscle (68).
Conventional Echo indices can hardly separate PM/DM patients with cardiac involvement from healthy controls. Except for the study of Wang et al., which detected significantly more left atrium enlargement and left ventricular diastolic dysfunction in PM/DM patients via conventional Echo (24), none of the remaining studies found statistically significant differences in conventional Echo indices (21,55,56,71-73).
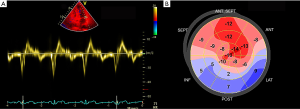
In recent years, new ultrasound technologies have been developed to assess cardiac morphology and performance in patients with IIMs. New Echo modalities can detect cardiac involvement more sensitively than conventional Echo, especially diastolic dysfunction (21,24,55,56,71-73). Both Guerra et al. and Zhong et al. demonstrated that the global longitudinal strain (GLS) of the left ventricle and right ventricle was significantly decreased in IIMs compared to the normal population (72,73). Stress Echo has not yet been introduced to PM/DM, but relevant studies have been carried out in other connective tissue diseases. Hirata et al. first used stress Echo to evaluate coronary flow reserve in systemic lupus erythaematosus (SLE) patients and proved altered coronary microvascular function in premenopausal women with SLE (74). The use of ultrasound enhancing agents (UEAs) has gradually become an integral component of Echo practice. Not only can they help to better measure function and structure, they can also realize perfusion imaging during stress Echo to visualize the presence or absence of an intact coronary microvasculature (75). Although no study has assessed cardiac involvement in PM/DM, UEAs can be of potential use in IIMs by visualizing impaired micro- or macrovascular circulation. In all, new imaging tests such as strain analysis, which allows a better assessment of cardiac morphology and performance by assessing regional cardiac mechanics and detecting subclinical myocardial dysfunctions that are still asymptomatic (Figure 2), have great advantages over conventional Echo indices in terms of subclinical cardiac involvement in IIM patients. The exact contributions of these new techniques still need further investigation.
Cardiac magnetic resonance
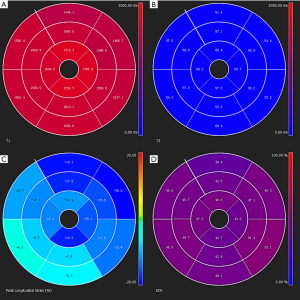
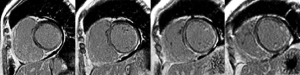
Cardiac magnetic resonance (CMR) is excellent for performing myocardial tissue characterization (52), which is useful for preclinical diagnosis of cardiac involvement in PM/DM (Figure 3). CMR has great advantages in different aspects. First, CMR can reveal silent myocardial involvement in PM/DM patients without any clinical evidence of cardiac involvement via late gadolinium enhancement (LGE) (Figure 4). A total of 16 IIM patients with no typical clinical symptoms, laboratory abnormalities or concomitant traditional cardiovascular risk factors were studied, and LGE was detected in 9 (56.3%) patients (76). Myocarditis was confirmed in another case with only mildly elevated cTnT, non-specific ECG changes and normal Echo, presenting as patchy mid-wall LGE in the basal aspect of the lateral and septal walls of the left ventricle (77). Second, the sensitivity of CMR in terms of detecting localized but not diffused myocarditis is also high (77). Third, CMR holds the possibility of uncovering myocarditis in the acute phase of PM/DM, whether symptomatic or asymptomatic, which was demonstrated in 20 PM/DM patients with a median disease duration of 20 days (78). Fourth, CMR can work as an indicator of treatment efficacy. It does not use ionizing radiation and allows for repeated scans over the disease duration (79). Allanore et al. found that in all PM/DM patients, CMR abnormalities were markedly reduced after 6 months of treatment (77,80). Finally, CMR has fewer limitations for technical reasons, such as operator-dependent bias, poor acoustic window, and increased breast size (81). In conclusion, CMR appears to be a very exciting diagnostic tool in PM/DM, although its use in early diagnosis and monitoring is still in its infancy, and more prospective controlled clinical trials are warranted. In the future, CMR may be a gatekeeper for invasive diagnostic methods such as X-ray coronary angiography and endomyocardial biopsy (52).
Positron emission tomography (PET) imaging
It has been 30 years since PET was pioneered for clinical use (82). PET has evolved to gain wider acceptance as a valuable modality for a variety of clinical conditions over the past 30 years, especially for detecting malignancies (83,84). Recently, it has been proven useful for diagnosing systemic inflammatory diseases such as rheumatoid arthritis (85). When it comes to PM/DM, PET imaging has not only been widely used for tumour detection, but detection of inflammatory myopathy and cardiomyopathy by PET imaging has also been a heated topic because inflammatory cells are also glucose-consuming, and fluorodeoxyglucose (FDG) is useful for detecting active inflammation (86-89). Li et al. concluded, after evaluating 38 IIM patients, that PET has great value in identifying malignancies, evaluating the status of myopathy, detecting interstitial lung disease (ILD), and predicting rapidly progressing ILD. Applying PET during the diagnosis and management of IIMs was recommended (90).
Furthermore, normal macrophages and lymphocytes have somatostatin receptors that are overexpressed (91). Accordingly, somatostatin receptor–targeted PET imaging with 68Ga-DOTANOC or 68Ga-DOTATOC can have better results in the detection of active cardiac lesions by eliminating the effects of glucose uptake by normal cardiomyocytes (Figure 5), which was supported by exploratory studies (92). Taking the rapid development of PET usage in IIMs (despite the main focus not being on cardiac involvement yet) and in inflammatory cardiomyopathies and new tracers such as 68Ga-DOTANOC or 68Ga-DOTATOC into consideration, it is reasonable to predict a promising future in the detection of myocardial involvement via PET.

The role of endomyocardial biopsy
Endomyocardial biopsy is the gold-standard technique for the diagnosis of myocarditis and inflammatory cardiomyopathy caused by viruses, drugs, toxic substances, or autoimmune conditions (93). In PM/DM, according to pathology reports, the findings of cardiac tissue resemble those in the skeletal muscle. Myocarditis (38%), focal myocardial fibrosis (22%) and conduction system fibrosis (20%) are the three most common manifestations (43). Small vessel disease, infarction and coronary sclerosis can also be seen (19,43,67,94). Endomyocardial biopsy can help discover some insidious causes of heart involvement in PM/DM, but more frequently, the result of biopsy might be negative because only myocardial fibrosis is found (95,96). The sensitivity of endomyocardial biopsy can be low because of the small sample size and the focal region of myocarditis (97). Additionally, endomyocardial biopsy is not routinely performed for the diagnosis of myocarditis considering safety reasons (98). The broad use of endomyocardial biopsy is limited because of the cost and accessibility of experienced centres (99). Thus, endomyocardial biopsy is an invasive technique that can be used only in strict clinical indications and has its own limitations. Targeted sampling needs to be improved with the help of the development of cardiac imaging (100).
Management
It is not surprising that the treatment strategy has been mainly based on immunosuppressive therapy and traditional heart medication (12,17). However, there are no available guidelines for treating cardiac involvement in myositis. It is reasonable that early, enhanced and sustained reduction of inflammatory status can help improve cardiac abnormalities because inflammatory changes are thought to be a cause of cardiac dysfunction leading to fibrosis (18,26,29). However, in clinical practice, it is still difficult and an empirical matter to decide the timing, intensity, duration, dosage and combination of immunosuppressive agents with glucocorticoid to stop or even reverse the fibrotic process. In addition, tailored, cardiac-specific therapy by a cardiologist is also needed. Several cases about the management of heart involvement in PM/DM, including both successful and failed cases, are listed as follows for reference.
Successful pharmacological treatment has been reported. Sharma et al. reported two patients who recovered from myocarditis, depressed biventricular function, and congestive heart failure after treatment with pulse-dose corticosteroid followed by oral prednisone and methotrexate and prednisone alone, respectively (101). In four patients taking high-dose corticosteroids and immunosuppressors for 6 months, the area of contrast enhancement and hypokinesia was markedly reduced in cardiac magnetic resonance (CMR) (80). Similarly, Péter et al. demonstrated that left ventricular and right ventricular systolic dysfunction improved significantly after the first 3 months of immunosuppressive therapy. The basic drug was high-dose corticosteroid, which was gradually tapered. Cyclosporine was added in patients with ILD and no improvements after taking steroids (21). In contrast, the CMR was still myocarditis (+) after 3 months of steroid treatment alone in one study by Mavrogeni and colleagues (78). Moreover, cardiac problems can occur even in patients in remission (102).
In case the steroids and first-line immunosuppressants is not well tolerated or not sufficiently effective, the use of intravenous immunoglobulin G (IVIG) has been supported in refractory PM and DM in clinical trials (103). Several case reports, case series and open-label trials have proven the efficacy of rituximab, a CD20 monoclonal antibody in patients with refractory myositis (104). Besides, other experimental treatment strategies are being carried out. Clinical and laboratory improvement has been demonstrated in some case reports and ongoing or small trials after using biological agents, including abatacept, tocilizumab, anakinra, sifalimumab and JAK inhibitors. But the data still require confirmation by larger, randomized and controlled studies (105). Anti-TNFα therapies are not supported because TNFα inhibitors might even induce myositis (106). Plasma exchange is not commonly used in myositis, but it may serve as a possible therapy in acute forms (107).
In conclusion, both traditional heart medication and steroids with or without immunosuppressive drugs are still cornerstones of the treatment. Standardized and effective therapeutic strategies, for both traditional agents and new emerging regimen still need to be verified by prospective studies in strictly selected patients.
Summary
PM and DM are autoimmune diseases that can lead to long-term disability and death, especially when accompanied with cardiac involvement. Although relatively difficult, recognizing early-stage cardiac involvement can help initiate effective treatment and potentially improve the prognosis. Increasing knowledge is gained on currently available auxiliary examinations, and their role in the diagnosis of PM/DM has been gradually revealed. With concomitantly increased knowledge regarding the pathogenesis of cardiac involvement in PM/DM, multiple immunosuppressive biological agents could enter clinical trials in the future. It is hopeful that this review will remind clinicians of the high prevalence and severity of cardiac involvement in PM/DM patients and inform them of the characteristics of currently available diagnostic approaches to realize a more timely and accurate diagnosis through the integration of various techniques.
Acknowledgments
Funding: This work was supported by the Beijing Natural Science Foundation (No.7192156 to Wei Chen).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-650). All authors report grants from Beijing Natural Science Foundation [No. 7192156 to WC] during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bazzani C, Cavazzana I, Ceribelli A, et al. Cardiological features in idiopathic inflammatory myopathies. J Cardiovasc Med (Hagerstown) 2010;11:906-11. [Crossref] [PubMed]
- Cavagna L, Monti S, Caporali R, et al. How I treat idiopathic patients with inflammatory myopathies in the clinical practice. Autoimmun Rev 2017;16:999-1007. [Crossref] [PubMed]
- Mandel DE, Malemud CJ, Askari AD. Idiopathic Inflammatory Myopathies: A Review of the Classification and Impact of Pathogenesis. Int J Mol Sci 2017;18:1084. [Crossref] [PubMed]
- Schmidt J. Current Classification and Management of Inflammatory Myopathies. J Neuromuscul Dis 2018;5:109-29. [Crossref] [PubMed]
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344-7. [Crossref] [PubMed]
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403-7. [Crossref] [PubMed]
- Tanimoto K, Nakano K, Kano S, et al. Classification criteria for polymyositis and dermatomyositis. J Rheumatol 1995;22:668-74. [PubMed]
- Troyanov Y, Targoff IN, Tremblay JL, et al. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore) 2005;84:231-49. [Crossref] [PubMed]
- Dimachkie MM, Barohn RJ. Idiopathic inflammatory myopathies. Semin Neurol 2012;32:227-36. [Crossref] [PubMed]
- Mavrogeni S, Sfikakis PP, Dimitroulas T, et al. Cardiac and muscular involvement in idiopathic inflammatory myopathies: noninvasive diagnostic assessment and the role of cardiovascular and skeletal magnetic resonance imaging. Inflamm Allergy Drug Targets 2014;13:206-16. [Crossref] [PubMed]
- Pipitone N. Value of MRI in diagnostics and evaluation of myositis. Curr Opin Rheumatol 2016;28:625-30. [Crossref] [PubMed]
- Lundberg IE. The heart in dermatomyositis and polymyositis. Rheumatology (Oxford) 2006;45 Suppl 4:iv18-21. [Crossref] [PubMed]
- Sultan SM, Ioannou Y, Moss K, et al. Outcome in patients with idiopathic inflammatory myositis: morbidity and mortality. Rheumatology (Oxford) 2002;41:22-6. [Crossref] [PubMed]
- Dankó K, Ponyi A, Constantin T, et al. Long-term survival of patients with idiopathic inflammatory myopathies according to clinical features: a longitudinal study of 162 cases. Medicine (Baltimore) 2004;83:35-42. [Crossref] [PubMed]
- Schiopu E, Phillips K, MacDonald PM, et al. Predictors of survival in a cohort of patients with polymyositis and dermatomyositis: effect of corticosteroids, methotrexate and azathioprine. Arthritis Res Ther 2012;14:R22. [Crossref] [PubMed]
- Van Gelder H, Charles-Schoeman C. The heart in inflammatory myopathies. Rheum Dis Clin North Am 2014;40:1-10. [Crossref] [PubMed]
- Zhang L, Wang GC, Ma L, et al. Cardiac involvement in adult polymyositis or dermatomyositis: a systematic review. Clin Cardiol 2012;35:686-91. [PubMed]
- Schwartz T, Diederichsen LP, Lundberg IE, et al. Cardiac involvement in adult and juvenile idiopathic inflammatory myopathies. RMD Open 2016;2:e000291. [Crossref] [PubMed]
- Haupt HM, Hutchins GM. The heart and cardiac conduction system in polymyositis-dermatomyositis: a clinicopathologic study of 16 autopsied patients. Am J Cardiol 1982;50:998-1006. [Crossref] [PubMed]
- Baughman KL. Clinical presentations of myocarditis. Heart Fail Clin 2005;1:363-76. [Crossref] [PubMed]
- Péter A, Balogh A, Szilagyi S, et al. Echocardiographic abnormalities in new-onset polymyositis/dermatomyositis. J Rheumatol 2015;42:272-81. [Crossref] [PubMed]
- Luyster FS, Kip KE, Buysse DJ, et al. Traditional and nontraditional cardiovascular risk factors in comorbid insomnia and sleep apnea. Sleep 2014;37:593-600. [Crossref] [PubMed]
- Diederichsen LP, Diederichsen AC, Simonsen JA, et al. Traditional cardiovascular risk factors and coronary artery calcification in adults with polymyositis and dermatomyositis: a Danish multicenter study. Arthritis Care Res (Hoboken) 2015;67:848-54. [Crossref] [PubMed]
- Wang H, Tang J, Chen X, et al. Lipid profiles in untreated patients with dermatomyositis. J Eur Acad Dermatol Venereol 2013;27:175-9. [Crossref] [PubMed]
- de Moraes MT, de Souza FH, de Barros TB, et al. Analysis of metabolic syndrome in adult dermatomyositis with a focus on cardiovascular disease. Arthritis Care Res (Hoboken) 2013;65:793-9. [Crossref] [PubMed]
- Diederichsen LP. Cardiovascular involvement in myositis. Curr Opin Rheumatol 2017;29:598-603. [Crossref] [PubMed]
- Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol 2012;27:1059-66. [Crossref] [PubMed]
- Fardet L, Feve B. Systemic glucocorticoid therapy: a review of its metabolic and cardiovascular adverse events. Drugs 2014;74:1731-45. [Crossref] [PubMed]
- Kim E, Cook-Mills J, Morgan G, et al. Increased expression of vascular cell adhesion molecule 1 in muscle biopsy samples from juvenile dermatomyositis patients with short duration of untreated disease is regulated by miR-126. Arthritis Rheum 2012;64:3809-17. [Crossref] [PubMed]
- Frantz S, Falcao-Pires I, Balligand JL, et al. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur J Heart Fail 2018;20:445-59. [Crossref] [PubMed]
- Taylor AJ, Wortham DC, Burge JR, et al. The heart in polymyositis: a prospective evaluation of 26 patients. Clin Cardiol 1993;16:802-8. [Crossref] [PubMed]
- Creus KK, De Paepe B, De Bleecker JL. Idiopathic inflammatory myopathies and the classical NF-kappaB complex: current insights and implications for therapy. Autoimmun Rev 2009;8:627-31. [Crossref] [PubMed]
- De Bleecker JL, Meire VI, Declercq W, et al. Immunolocalization of tumor necrosis factor-alpha and its receptors in inflammatory myopathies. Neuromuscul Disord 1999;9:239-46. [Crossref] [PubMed]
- Stern R, Godbold JH, Chess Q, et al. ECG abnormalities in polymyositis. Arch Intern Med 1984;144:2185-9. [Crossref] [PubMed]
- Nagaraju K, Raben N, Loeffler L, et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc Natl Acad Sci U S A 2000;97:9209-14. [Crossref] [PubMed]
- Englund P, Nennesmo I, Klareskog L, et al. Interleukin-1alpha expression in capillaries and major histocompatibility complex class I expression in type II muscle fibers from polymyositis and dermatomyositis patients: important pathogenic features independent of inflammatory cell clusters in muscle tissue. Arthritis Rheum 2002;46:1044-55. [Crossref] [PubMed]
- Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol 1985;37:269-390. [Crossref] [PubMed]
- Wang J, Okazaki IM, Yoshida T, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol 2010;22:443-52. [Crossref] [PubMed]
- Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016;375:1749-55. [Crossref] [PubMed]
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-82. [Crossref] [PubMed]
- Hochberg MC, Feldman D, Stevens MB. Adult onset polymyositis/dermatomyositis: an analysis of clinical and laboratory features and survival in 76 patients with a review of the literature. Semin Arthritis Rheum 1986;15:168-78. [Crossref] [PubMed]
- Bohan A, Peter JB, Bowman RL, et al. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore) 1977;56:255-86. [Crossref] [PubMed]
- Gupta R, Wayangankar SA, Targoff IN, et al. Clinical cardiac involvement in idiopathic inflammatory myopathies: a systematic review. Int J Cardiol 2011;148:261-70. [Crossref] [PubMed]
- Sasaki H, Kohsaka H. Current diagnosis and treatment of polymyositis and dermatomyositis. Mod Rheumatol 2018;28:913-21. [Crossref] [PubMed]
- Coley W, Rayavarapu S, Pandey GS, et al. The molecular basis of skeletal muscle weakness in a mouse model of inflammatory myopathy. Arthritis Rheum 2012;64:3750-9. [Crossref] [PubMed]
- Agewall S, Giannitsis E, Jernberg T, et al. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J 2011;32:404-11. [Crossref] [PubMed]
- Schwarzmeier JD, Hamwi A, Preisel M, et al. Positive troponin T without cardiac involvement in inclusion body myositis. Hum Pathol 2005;36:917-21. [Crossref] [PubMed]
- Cox FM, Delgado V, Verschuuren JJ, et al. The heart in sporadic inclusion body myositis: a study in 51 patients. J Neurol 2010;257:447-51. [Crossref] [PubMed]
- Bodor GS, Survant L, Voss EM, et al. Cardiac troponin T composition in normal and regenerating human skeletal muscle. Clin Chem 1997;43:476-84. [Crossref] [PubMed]
- Hughes M, Lilleker JB, Herrick AL, et al. Cardiac troponin testing in idiopathic inflammatory myopathies and systemic sclerosis-spectrum disorders: biomarkers to distinguish between primary cardiac involvement and low-grade skeletal muscle disease activity. Ann Rheum Dis 2015;74:795-8. [Crossref] [PubMed]
- Chen F, Peng Y, Chen M. Diagnostic Approach to Cardiac Involvement in Idiopathic Inflammatory Myopathies. Int Heart J 2018;59:256-62. [Crossref] [PubMed]
- Fishbein MC, Wang T, Matijasevic M, et al. Myocardial tissue troponins T and I. An immunohistochemical study in experimental models of myocardial ischemia. Cardiovasc Pathol 2003;12:65-71. [Crossref] [PubMed]
- Ungprasert P, Suksaranjit P, Spanuchart I, et al. Risk of coronary artery disease in patients with idiopathic inflammatory myopathies: a systematic review and meta-analysis of observational studies. Semin Arthritis Rheum 2014;44:63-7. [Crossref] [PubMed]
- Rai SK, Choi HK, Sayre EC, et al. Risk of myocardial infarction and ischaemic stroke in adults with polymyositis and dermatomyositis: a general population-based study. Rheumatology (Oxford) 2016;55:461-9. [PubMed]
- Plazak W, Kopec G, Tomkiewicz-Pajak L, et al. Heart structure and function in patients with generalized autoimmune diseases: echocardiography with tissue Doppler study. Acta Cardiol 2011;66:159-65. [Crossref] [PubMed]
- Lu Z, Wei Q, Ning Z, et al. Left ventricular diastolic dysfunction -- early cardiac impairment in patients with polymyositis/dermatomyositis: a tissue Doppler imaging study. J Rheumatol 2013;40:1572-7. [Crossref] [PubMed]
- Tansley S, Gunawardena H. The evolving spectrum of polymyositis and dermatomyositis--moving towards clinicoserological syndromes: a critical review. Clin Rev Allergy Immunol 2014;47:264-73. [Crossref] [PubMed]
- Imbert-Masseau A, Hamidou M, Agard C, et al. Antisynthetase syndrome. Joint Bone Spine 2003;70:161-8. [Crossref] [PubMed]
- Zhang L, Wu G, Gao D, et al. Factors Associated with Interstitial Lung Disease in Patients with Polymyositis and Dermatomyositis: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0155381. [Crossref] [PubMed]
- Fiorentino D, Chung L, Zwerner J, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol 2011;65:25-34. [Crossref] [PubMed]
- Ghirardello A, Bassi N, Palma L, et al. Autoantibodies in polymyositis and dermatomyositis. Curr Rheumatol Rep 2013;15:335. [Crossref] [PubMed]
- Love LA, Leff RL, Fraser DD, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991;70:360-74. [Crossref] [PubMed]
- Kao AH, Lacomis D, Lucas M, et al. Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum 2004;50:209-15. [Crossref] [PubMed]
- Albayda J, Khan A, Casciola-Rosen L, et al. Inflammatory myopathy associated with anti-mitochondrial antibodies: A distinct phenotype with cardiac involvement. Semin Arthritis Rheum 2018;47:552-6. [Crossref] [PubMed]
- Buchpiguel CA, Roizemblatt S, Pastor EH, et al. Cardiac and skeletal muscle scintigraphy in dermato- and polymyositis: clinical implications. Eur J Nucl Med 1996;23:199-203. [Crossref] [PubMed]
- Behan WM, Behan PO, Gairns J. Cardiac damage in polymyositis associated with antibodies to tissue ribonucleoproteins. Br Heart J 1987;57:176-80. [Crossref] [PubMed]
- Denbow CE, Lie JT, Tancredi RG, et al. Cardiac involvement in polymyositis: a clinicopathologic study of 20 autopsied patients. Arthritis Rheum 1979;22:1088-92. [Crossref] [PubMed]
- Gottdiener JS, Sherber HS, Hawley RJ, et al. Cardiac manifestations in polymyositis. Am J Cardiol 1978;41:1141-9. [Crossref] [PubMed]
- Benbassat J, Gefel D, Larholt K, et al. Prognostic factors in polymyositis/dermatomyositis. A computer-assisted analysis of ninety-two cases. Arthritis Rheum 1985;28:249-55. [Crossref] [PubMed]
- Agrawal CS, Behari M, Shrivastava S, et al. The heart in polymyositis-dermatomyositis. J Neurol 1989;236:249-50. [Crossref] [PubMed]
- Diederichsen LP, Simonsen JA, Diederichsen AC, et al. Cardiac Abnormalities in Adult Patients With Polymyositis or Dermatomyositis as Assessed by Noninvasive Modalities. Arthritis Care Res (Hoboken) 2016;68:1012-20. [Crossref] [PubMed]
- Zhong Y, Bai W, Xie Q, et al. Cardiac function in patients with polymyositis or dermatomyositis: a three-dimensional speckle-tracking echocardiography study. Int J Cardiovasc Imaging 2018;34:683-93. [PubMed]
- Guerra F, Gelardi C, Capucci A, et al. Subclinical Cardiac Dysfunction in Polymyositis and Dermatomyositis: A Speckle-tracking Case-control Study. J Rheumatol 2017;44:815-21. [Crossref] [PubMed]
- Hirata K, Kadirvelu A, Kinjo M, et al. Altered coronary vasomotor function in young patients with systemic lupus erythematosus. Arthritis Rheum 2007;56:1904-9. [Crossref] [PubMed]
- Porter TR, Mulvagh SL, Abdelmoneim SS, et al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J Am Soc Echocardiogr 2018;31:241-74. [Crossref] [PubMed]
- Mavrogeni S, Douskou M, Manoussakis MN. Contrast-enhanced CMR imaging reveals myocardial involvement in idiopathic inflammatory myopathy without cardiac manifestations. JACC Cardiovasc Imaging 2011;4:1324-5. [Crossref] [PubMed]
- Toong C, Puranik R, Adelstein S. Use of cardiac MR imaging to evaluate the presence of myocarditis in autoimmune myositis: three cases. Rheumatol Int 2012;32:779-82. [Crossref] [PubMed]
- Mavrogeni S, Bratis K, Karabela G, et al. Myocarditis during acute inflammatory myopathies: evaluation using clinical criteria and cardiac magnetic resonance imaging. Int J Cardiol 2013;164:e3-4. [Crossref] [PubMed]
- Mavrogeni S, Bratis K, Kolovou G. Cardiac magnetic resonance for early detection and risk stratification of patients with non-compaction cardiomyopathy. Eur J Heart Fail 2011;13:1153-4; author reply 1154. [Crossref] [PubMed]
- Allanore Y, Vignaux O, Arnaud L, et al. Effects of corticosteroids and immunosuppressors on idiopathic inflammatory myopathy related myocarditis evaluated by magnetic resonance imaging. Ann Rheum Dis 2006;65:249-52. [Crossref] [PubMed]
- Chelu RG, van den Bosch AE, van Kranenburg M, et al. Qualitative grading of aortic regurgitation: a pilot study comparing CMR 4D flow and echocardiography. Int J Cardiovasc Imaging 2016;32:301-7. [Crossref] [PubMed]
- Gould KL, Schelbert HR, Phelps ME, et al. Noninvasive assessment of coronary stenoses with myocardial perfusion imaging during pharmacologic coronary vasodilatation. V. Detection of 47 percent diameter coronary stenosis with intravenous nitrogen-13 ammonia and emission-computed tomography in intact dogs. Am J Cardiol 1979;43:200-8. [Crossref] [PubMed]
- McArdle B, Dowsley TF, Cocker MS, et al. Cardiac PET: metabolic and functional imaging of the myocardium. Semin Nucl Med 2013;43:434-48. [Crossref] [PubMed]
- Tateyama M, Fujihara K, Misu T, et al. Clinical values of FDG PET in polymyositis and dermatomyositis syndromes: imaging of skeletal muscle inflammation. BMJ Open 2015;5:e006763. [Crossref] [PubMed]
- Kubota K, Ito K, Morooka M, et al. FDG PET for rheumatoid arthritis: basic considerations and whole-body PET/CT. Ann N Y Acad Sci 2011;1228:29-38. [Crossref] [PubMed]
- Gotthardt M, Bleeker-Rovers CP, Boerman OC, et al. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med 2010;51:1937-49. [PubMed]
- Mahmood S, Rodriguez Martinez de Llano S. 18F-FDG PET detection of unknown primary malignancy in dermatomyositis. Clin Nucl Med 2012;37:e204-5. [Crossref] [PubMed]
- Morita Y, Kuwagata S, Kato N, et al. 18F-FDG PET/CT useful for the early detection of rapidly progressive fatal interstitial lung disease in dermatomyositis. Intern Med 2012;51:1613-8. [Crossref] [PubMed]
- Saraste A, Knuuti J. PET imaging in heart failure: the role of new tracers. Heart Fail Rev 2017;22:501-11. [Crossref] [PubMed]
- Li Y, Zhou Y, Wang Q. Multiple values of (18)F-FDG PET/CT in idiopathic inflammatory myopathy. Clin Rheumatol 2017;36:2297-305. [Crossref] [PubMed]
- Lapa C, Reiter T, Kircher M, et al. Somatostatin receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: an initial comparison to cardiac MRI. Oncotarget 2016;7:77807-14. [Crossref] [PubMed]
- Gormsen LC, Haraldsen A, Kramer S, et al. A dual tracer (68)Ga-DOTANOC PET/CT and (18)F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res 2016;6:52. [Crossref] [PubMed]
- Dominguez F, Kuhl U, Pieske B, et al. Update on Myocarditis and Inflammatory Cardiomyopathy: Reemergence of Endomyocardial Biopsy. Rev Esp Cardiol (Engl Ed) 2016;69:178-87. [Crossref] [PubMed]
- Bazhanov NN, Khitrov AN, Nasonov EL, et al. Klin Med (Mosk) 1998;76:32-5. [Cardiac rhythm and conduction disorders in polymyositis and dermatomyositis]. [PubMed]
- Riemekasten G, Opitz C, Audring H, et al. Beware of the heart: the multiple picture of cardiac involvement in myositis. Rheumatology (Oxford) 1999;38:1153-7. [Crossref] [PubMed]
- Sénéchal M, Crete M, Couture C, et al. Myocardial dysfunction in polymyositis. Can J Cardiol 2006;22:869-71. [Crossref] [PubMed]
- Birnie DH, Kandolin R, Nery PB, et al. Cardiac manifestations of sarcoidosis: diagnosis and management. Eur Heart J 2017;38:2663-70. [PubMed]
- Pophal SG, Sigfusson G, Booth KL, et al. Complications of endomyocardial biopsy in children. J Am Coll Cardiol 1999;34:2105-10. [Crossref] [PubMed]
- Heymans S, Eriksson U, Lehtonen J, et al. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J Am Coll Cardiol 2016;68:2348-64. [Crossref] [PubMed]
- Unterberg-Buchwald C, Ritter CO, Reupke V, et al. Targeted endomyocardial biopsy guided by real-time cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2017;19:45. [Crossref] [PubMed]
- Sharma K, Orbai AM, Desai D, et al. Brief report: antisynthetase syndrome-associated myocarditis. J Card Fail 2014;20:939-45. [Crossref] [PubMed]
- Danieli MG, Gelardi C, Guerra F, et al. Cardiac involvement in polymyositis and dermatomyositis. Autoimmun Rev 2016;15:462-5. [Crossref] [PubMed]
- Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med 1993;329:1993-2000. [Crossref] [PubMed]
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology (Oxford) 2017;56:26-36. [Crossref] [PubMed]
- Glaubitz S, Zeng R, Schmidt J. New insights into the treatment of myositis. Ther Adv Musculoskelet Dis 2020;12:1759720X19886494.
- Ishikawa Y, Yukawa N, Ohmura K, et al. Etanercept-induced anti-Jo-1-antibody-positive polymyositis in a patient with rheumatoid arthritis: a case report and review of the literature. Clin Rheumatol 2010;29:563-6. [Crossref] [PubMed]
- Cozzi F, Marson P, Pigatto E, et al. Plasma-exchange as a "rescue therapy" for dermato/polymyositis in acute phase. Experience in three young patients. Transfus Apher Sci 2015;53:368-72. [Crossref] [PubMed]


