
Drug resistance of healthcare-associated pathogenic bacteria and carbapenem-resistant Acinetobacter baumannii homology in the general intensive care unit
Introduction
Healthcare-associated infection (HAI) is a threat to medical quality in most healthcare facilities in both developed and developing countries. The incidence rate of HAI is around 5–10%; therefore, controlling and reducing HAI has become a significant global public health question (1,2). Since patients in an intensive care unit (ICU) have serious conditions like chronic disease, they undergo invasive operations and have low immunity, and are therefore more likely to contract HAI than other patients. One study showed that the risk of HAI in the ICU is 3 times greater than that in the ordinary medical unit (3), and the ICU is a critical department in HAI surveillance (4). Acinetobacter baumannii (CR-AB) is a Gram-negative bacillus and has proven to be the main pathogen causing HAI. CR-AB is the most common non-fermentative bacteria which causes HAI. Its drug resistance has become increasingly severe, and is highly likely to be the main cause of HAI outbreak (5,6). CR-AB infection results in it being widely distributed in the environment of the hospital. It can further infect different sites like the urinary tract, wounds, endocardium, lungs, and blood, after longer lengths of stay (7). Carbapenem-resistant CR-AB, the most common kind of multi-drug resistant CR-AB, can easily spread and will cause intractable infection. According to an American report, 49.5% of CR-AB cases are carbapenem-resistant. CR-AB has a significant relationship with CR-AB infection death (adjusted OR: 2.49, 95% CI: 1.61–3.84). Several countries like France, Germany, Greece, and Italy have reported CR-AB outbreaks. HAI pathogen distribution and drug resistance in general ICUs of the Inner Mongolia region were detected from January to May 2019 in 12 tertiary general hospitals. In total, 63 strains of non-repetitive CR-AB were isolated from ICU patients, healthcare workers (HCW), and the environment. Pulsed-field gel electrophoresis (PFGE) and homology analysis of the 63 strains were completed to provide evidence for CR-AB infection treatment and prevention.
Method
Bacteria strain source
- Clinical sample: Phlegm, blood, urine, bile, and drainage from the central venous catheter (CVC), wound surface, enterocoelia, and thoracic cavity were collected from HAI patients from the general ICU in 12 general tertiary hospitals from January 1, 2019, to May 31, 2019. Repeated bacteria strains from the same site of the same patient were removed, while any CR-AB strains were kept.
- Environment sample: CR-AB strains from the ICU environment, object surface, air, and the hands of healthcare workers were collected during the same time period mentioned above.
- Quality control sample: Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Streptococcus pneumoniae ATCC 49619, and Haemophilus influenzae ATCC 49247 were provided by the laboratory department in Inner Mongolia People’s Hospital. PFGE was conducted using standard a salmonella strain (H9812) as a quality control strain, and was provided by the Inner Mongolia Center for Disease Control.
- Sample source: Samples were collected from 12 general tertiary hospitals distributed in 11 cities in Inner Mongolia Autonomous Region, covering almost all areas in the Inner Mongolia Autonomous Region. Inner Mongolia is found in the north part of China, and is the third-largest province in China. The east–west distance of Inner Mongolia is nearly 1,700 km.
Main reagent
The main reaguens used were Mueller-Hinton (MH), Salmonella Shigella (SS), Columbia blood, and Haemophilus selective chocolate agar culture medium; biphase blood culture; blood pathogen culture flask; Gram stain; identification and antimicrobial susceptibility test reagent; antibiotic susceptibility test discs; agarose (Seakem Gold Agarose); protease K; and restriction enzyme Xba I, Apa I, and GelRed.
Main device
The main devices used were blood culture system, Densicheck, microbial detection system, PFGE, and gel imaging system.
Strain identification and antimicrobial susceptibility test
A dominant bacteria strain from the culture medium was chosen according to the growth shape. VITEK2-compact Microbial detection system was used to conduct strain identification and antimicrobial susceptibility tests by matching reagents. Some individual strains were tested using an antibiotic disc to conduct a Kirby-Bauer (K-B) antimicrobial susceptibility test. Judgments for the quality control of the results were according to the 2016 recommended criteria of the The Clinical and Laboratory Standards Institute (CLSI) (8).
PFGE procedure
CR-AB strains were isolated from samples of healthcare workers, HAI patients, and the environment to conduct the PFGE test. Intructions for conducting the PFGE procedure were as follows: (I) revive strain (kept at -80 °C); (II) embed the bacteria in gel and use the corresponding Apa I endonuclease to restrict enzyme digestion on the DNA site; (III) change the small-sized gel block, including the DNA fragment, into a bigger sized gel block using 1.5% agarose gel; (IV) place a bigger sized gel block into 0.5 × TBE buffer solution to conduct PFGE; (V) dye, rinse, and image the gel block for observation and preservation.
Result determination
The National Molecular Subtyping Network for Bacterial Pathogens Surveillance (PulseNet China) software was used to recognize and dispose of the PFGE electrophoretic strip diagram. Electrophoretic strips of the test strain were located and adjusted by H9812 strain molecular weight standard, and manual adjustment was conducted when necessary. Determination of results was done according to the recommendations of Tenover (9) and Talon (10). The similarity coefficient was used to biotype from different strains. When gene homology ≥74%, 2 strains were regarded as the same cluster; when gene homology ≥85%, 2 strains were regarded as the same clone strain.
Statistical analysis
WHONET 5.6 software was used to conduct drug resistance analysis of the main pathogen bacteria, Excel 2010 was used for data statistics, and SPSS22.0 was used to conduct data analysis. Ratio (%) was used to express enumeration data, χ2 tests or Fisher’s exact test were used to comparison among groups, and P values <0.05 were regarded as statistically significant. PulseNet China software was used to analyze the PFGE image.
Ethical approval
The study was approved by the institutional ethics committee/ethics board of Inner Mongolia People’s Hospital (No. 202000203L). Since this study is not involve any patient’s personal information and the samples used in this study were after used samples from hospital lab, informed consent is not require in this study.
Result
Type and distribution of pathogen bacteria
A total of 696 strains of pathogen bacteria were isolated from HAI patients in the ICU of 12 general tertiary hospitals in the Inner Mongolia region from January to May 2018. Among them were included 89 strains from Inner Mongolia People’s Hospital, 79 strains from Second Hospital of Chifeng, 67 strains from the First Affiliated Hospital of Baotou Medical College, 57 strains from the Second Affiliated Hospital of Baotou Medical College, 77 strains from Tongliao City Hospital, 75 strains from the Central Hospital of Wulanchabu, 53 strains from Bayannur Hospital, 26 strains from Ordos Central Hospital, 21 strains from the People’s Hospital of Wuhai, 23 strains from the Hospital of XilinGol, 52 strains from the People’s Hospital of Hinggan League, and 77 strains from the Inner Mongolia Forestry General Hospital. Among them, gram-negative bacteria constituted 63.07% (439/696) of the total, and the main bacteria were CR-AB, Klebsiella pneumoniae, and Pseudomonas aeruginosa; gram-positive bacteria constituted 22.13% (154/696) of the total, and the main bacteria were Enterococcus faecium and Staphylococcus aureus; fungus constituted 14.80% (103/696) of the total, and the main strain was Candida albicans. The top 6 most common HAI bacteria were CR-AB, Klebsiella pneumonia, Pseudomonas aeruginosa, Enterococcus faecium, Escherichia coli, and Staphylococcus aureus (Table 1).
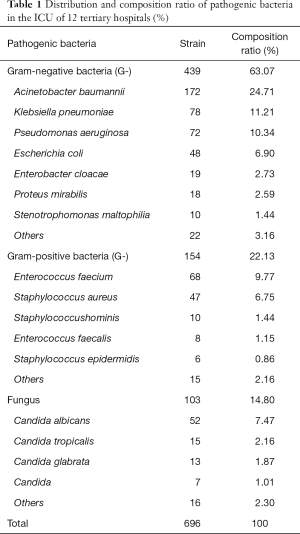
Full table
In total, 696 strains of pathogen bacteria were detected from sputum samples (41.09%, 286/696), midstream urine samples (23.13%, 161/696), blood samples (17.82%, 124/696), CVC samples (4.60%, 32/696), secretion samples (4.31%, 30/696), and others (Table 2).
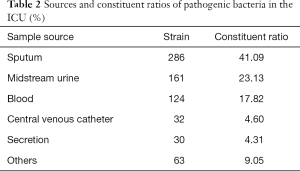
Full table
Drug resistance of major pathogenic bacteria
Drug resistance of CR-AB and Pseudomonas aeruginosa
CR-AB had low drug resistance to levofloxacin (39.53%) and high drug resistance (above 80%) to other known antibiotics. CR-AB showed pan-drug resistance to piperacillin, ampicillin, cefuroxime, cefazolin, cefotetan, and macrodantin. Pseudomonas aeruginosa had low drug resistance (below 10%) to amikacin (5.71%) and tobramycin (5.56%), and showed pan-drug resistance to ampicillin, ampicillin-sulbactam, cefuroxime, cefazolin, ceftriaxone, and cefotetan. CR-AB’s drug resistance rate to piperacillin, piperacillin-tazobactam, ceftazidime, cefepime, imipenem, tobramycin, gentamicin, levofloxacin, and ciprofloxacin was higher than that of pseudomonas aeruginosa (P<0.05). Furthermore, CR-AB’s drug resistance rate to ampicillin-sulbactam, which are pediatric compound sulfamethoxazole tablets, was lower than that of Pseudomonas aeruginosa (P<0.05). Also, CR-AB drug’s resistance rate to ceftriaxone had no significant statistical difference compared to that of pseudomonas aeruginosa (χ2 =0.320, P=0.572) (Table 3).
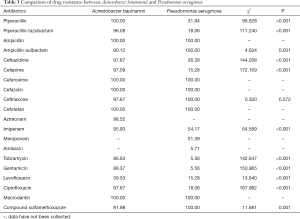
Full table
Drug resistance of Klebsiella pneumoniae and Escherichia coli
Klebsiella pneumoniae showed low drug resistance (below 50%) to piperacillin-tazobactam, cefotetan, imipenem, meropenem, amikacin, and tobramycin, but had high (above 60%) drug resistance to other antibiotics. It especially showed pan-drug resistance to ampicillin. Escherichia coli showed low drug resistance (below 20%) to piperacillin-tazobactam, cefotetan, imipenem, meropenem, amikacin, tobramycin, and macrodantin. Klebsiella pneumoniae’s drug resistance rate to piperacillin-tazobactam, ceftazidime, cefotetan, imipenem, meropenem, amikacin, tobramycin, and macrodantin was higher than that of Escherichia coli (P<0.05) (Table 4).
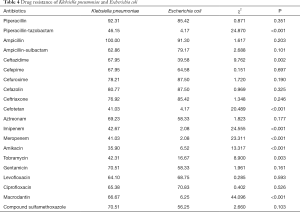
Full table
Drug resistance of Enterococcus faecium and Staphylococcus aureus
Among all gram-positive bacteria, Enterococcus faecium and Staphylococcus aureus constituted the largest portion of all bacteria. Enterococcus faecium was sensitive to tigecycline and linezolid, and showed pan-drug resistance to ampicillin, penicillin, clindamycin, levofloxacin, ciprofloxacin, and moxifloxacin. Meanwhile, Staphylococcus aureus was sensitive to tigecycline, vancomycin, macrodantin, and linezolid, and showed pan-drug resistance to cefoxitin. Enterococcus faecium’s drug resistance rate for erythrocin, clindamycin, levofloxacin, moxifloxacin, and macrodantin was higher than that of staphylococcus aureus (P<0.05) (Table 5).
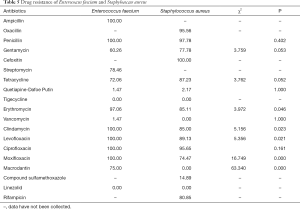
Full table
Detection result of CR-AB
From January to May 2019, a total of 63 strains of non-repetitive CR-AB bacteria were collected from HAI patients and the environment of 12 ICUs; 46 strains were collected from patient samples (phlegm sample), and 17 strains were collected from environment samples (Table 6).
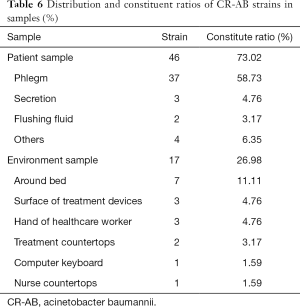
Full table
PFGE typing of CR-AB
The 63 strains of CR-AB collected from HAI patient and the environment of 12 ICUs were used to conduct PFGE, and 20–30 strips were generated (Figure 1). BioNumerics software was used to analyze PFGE electrophoretograms. Based on the principle of 100% similarity regarded as the same PFGE type, a total of 62 types of strip were generated, and only BM17 and BM30 were 100% the same. A 74% similarity coefficient was used to conduct UPGMA cluster analysis, and resulted in 16 clusters (A–P). Finally, 16 clusters were scatter distributed: K, L, and N types constituted the majority (more than 9 strains), I type included 5 strains, and other types only included 1–3 strains. Based on the Tenover criteria which states that >85% similarity in strips should be regarded as the same strain, we can conclude from cluster analysis (Figure 2) that many strains were the same. For example, BM39 and BM41 were from the same patient sample from the Central Hospital of Wulanchabu, while BM49 and BM50 were from the patient phlegm sample and surface sample of a ventilator, respectively.
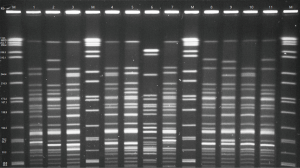
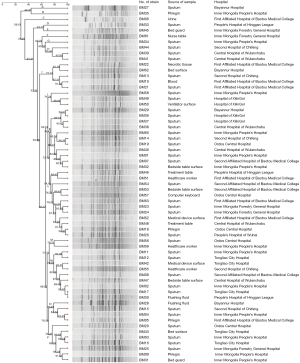
Discussion
HAI is an infection caused by pathogenic microorganisms and microorganisms, which usually do not cause harm to human health but eventually result in human infection and clinical symptoms in different ways. Following the increasing amount of newly discovered antibiotics, death caused by HAI has decreased sharply. Nevertheless, multi-drug resistant organisms (MDRO) have subsequently raised a considerable challenge to healthcare workers. Thus, we need to understand the local distribution of pathogens in the ICU and their drug resistance in order to choose better clinical antibiotics. According to the result of this study, gram-negative bacteria constituted the majority of the 696 strains of pathogen bacteria detected from the ICU in 12 general tertiary hospitals from January to May 2019. Non-fermentative CR-AB had the highest detection rate, which is the same as a result from the China Antimicrobial Resistance Surveillance System (11-12). Non-fermentative bacteria are conditioned pathogens that generally exist in the environment. Non-fermentative bacteria can form colonies in humans with no clinical symptoms, and can turn into pathogenic bacteria when immunity decreases.
The sputum sample constituted 41.09% of the sample total, which shows that the respiratory tract is the most common infection site in the ICU, which is similar to other findings in China (13). A possible reason for this could be that the critical condition of the patients in ICU may hve compromised consciousness, trachea intubation, tracheostomy, ventilator usage, venipuncture, or sputum aspiration damaging mucous epithelia of the respiratory tract; all contributing to declined immune function. For the resons outlined above, it is easier for bacteria to adhere to and colonize the respiratory tract (14). The 6 most common bacteria for HAI patients in the ICU were CR-AB, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterococcus faecium, Escherichia coli, and Staphylococcus aureus; this composition differed from that in Chen’s survey (15), while the ICU bacteria distribution differed from that of the other regions in China.
CR-AB and Pseudomonas aeruginosa are non-fermentative gram-negative bacteria. CR-AB can exist in the skin, respiratory tract, and urinary tract of healthy people. CR-AB showed pan-drug resistance to several kinds of antibiotics in this study. For example, it had resistance to piperacillin, ampicillin, cefuroxime, cefazolin, cefotetan, and macrodantin. Furthermore. It showed a 39.53% drug resistance to levofloxacin, which means levofloxacin is an excellent antibiotic for the clinical treatment of CR-AB infection. One study examined single or combined use of tigecycline in CR-AB infection treatment, which resulted in the appearance of tigecycline resistant strain A and B (16). Another study also showed that CR-AB will be increasingly drug-resistant to carbapenems and other antibiotics. All patients in the ICU need a ventilator or intubation tube, which can easily lead to Pseudomonas aeruginosa infection. According to drug sensitivity tests, Pseudomonas aeruginosa is sensitive to amikacin and tobramycin, with a drug resistance rate of 5.71% and 5.56%, respectively. Therefore, treatment of Pseudomonas aeruginosa infection should potentially first involve piperacillin, piperacillin, tazobactam, and ceftazidime and avoid penicillin or cephalosporin. CR-AB, has a more severe condition of drug resistance than Pseudomonas aeruginosa, meaning it is more likely to have drug resistance to these kinds of antibiotics. Klebsiella pneumoniae showed pan-drug resistance to ampicillin, and lower than 50% drug resistance to piperacillin, tazobactam, cefotetan, imipenem, meropenem, amikacin, and tobramycin; Escherichia coli showed high sensitivity to piperacillin, tazobactam, cefotetan, imipenem, meropenem, amikacin, tobramycin, and macrodantin. For the 8 kinds of antibiotics mentioned above, Klebsiella pneumoniae had a higher drug resistance than Escherichia coli.
Among gram-positive bacteria, Enterococcus faecium and Staphylococcus aureus constituted the largest portion, which is consistent with a study (17) showing that staphylococcus aureus is a common kind of HAI pathogen bacteria, widely distributed in patient’s skin and the environment of the inpatient ward. Staphylococcus aureus showed pan-drug resistance to cefoxitin, but no drug-resistant strain was found for tigecycline, vancomycin, macrodantin, or linezolid. Enterococcus faecium, on the other hand, was sensitive to tigecycline and linezolid but pan-drug resistant to ampicillin, penicillin, clindamycin, levofloxacin, ciprofloxacin, and moxifloxacin. We found that Enterococcus faecium had a higher drug resistance than Staphylococcus aureus for clindamycin, levofloxacin, moxifloxacin, and macrodantin.
The 63 strains of CR-AB bacteria collected in this study were used to conduct homology analysis; the PFGE test figure was input into PulseNet China software to obtain a cluster analysis figure. The cluster analysis figure showed that CR-AB is sporadically across the Inner Mongolia region, with a cross-infection occurring between the patient and the environment. To better prevent cross-infection, isolating infected patients, cleaning and disinfecting the environment, and strengthening the hand hygiene of healthcare workers will have a beneficial effect. Especially when considering healthcare worker’s low compliance of hand hygiene, the focus should be surveillance and prevention, since hospital cross-infection is mostly caused by contaminated hands. Therefore, it is essential to strengthen the awareness of aseptic operation and focus on training of standard operation for healthare workers. Timely and strict disinfectant medical devices (18), rational use of antibiotics, personal protection of healthcare workers, and medical waste management could also prevent outbreaks and reduce the number of HAI cases (19-21).
Conclusions
In conclusion, HAI pathogenic bacteria in the ICUs of Inner Mongolia have multi-drug resistance. G-bacteria were the primary pathogens of HAI in the ICU, which is similar to findings in other regions (22,23). Therefore, the connection between the HAI management department and the clinical microbiology lab should be strengthened. Healthcare workers should promptly and periodically investigate any pathogenic bacteria of inpatients to dynamically observe drug resistance of ICU pathogen bacteria. Clinical doctors could choose antibiotics according to a drug sensitivity test which could simultaneously prevent a drug-resistant bacteria epidemic. It is necessary to ensure that the right-hand hygiene method is used, while strict adherence to aseptic operation and immediate disinfection of environment and air can effectively reduce cross-infection between patients, further preventing CR-AB outbreak in healthcare facilities.
Acknowledgments
Funding: This work was supported by the Department of Science & Technology of Inner Mongolia (No. 2017MS(LH)0845), the Department of Human Resources and Social Security of Inner Mongolia, the State Key Laboratory for Infectious Disease Prevention and Control of the Chinese Center for Disease control and Prevention [2019SKLID305], the Infection Prevention and Control Research Fund Administration Commission of the China Geriatric Society (No. GRYJ-LRK2018021), and the Health Commission of Inner Mongolia (No. 201703006).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-19-632
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-632). WL, YY, KZ, YH, HL, YJ, HX, BX, HB, YZ and TG report grants from Department of Science & Technology of Inner Mongolia, Department of Human Resources and Social Security of Inner Mongolia, State key laboratory for infectious disease prevention and control of Chinese Center for disease control and prevention, Infection Prevention and Control Research Fund Administration Commission of China Geriatric Society, and Health Comission of Inner Mongolia during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee/ethics board of Inner Mongolia People’s Hospital (No. 202000203L). Since this study is not involve any patient’s personal information and the samples used in this study were after used samples from hospital lab, informed consent is not require in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pan HP, Chu CJ, Chen LH, et al. Clinical distribution and antimicrobial resistance of pathogens causing healthcare-associated infection in a comprehensive hospital. Chinese Journal of Infection Control 2017;16:225-8.
- Gong Y, Shen X, Huang G, et al. Epidemiology and resistance features of Acinetobacter baumannii isolates from the ward environment and patients in the burn ICU of a Chinese hospital. J Microbiol 2016;54:551-8. [Crossref] [PubMed]
- Mahamat A, Bertrand X, Moreau B, et al. Clinical epidemiology and resistance mechanisms of carbapenem-resistant Acinetobacter baumannii, French Guiana, 2008–2014. Int J Antimicrob Agents 2016;48:51-5. [Crossref] [PubMed]
- Vincent JL, Sakr Y, Singer M, et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020;323:1478-87. [Crossref] [PubMed]
- Hu FP, Guo Y, Zhu DM, et al. Antimicrobial resistance profile of clinical isolates in hospitals across China:report from the CHINET Surveillance Program, 2017. Chinese Journal of Infection and Chemotherapy 2018;18:241-51.
- Cao QQ, Yu LX, Wang HP. Study of clinical distribution and antimicrobial resistance of Acinetobacter baumannii causing nosocomial infections. Chinese Journal of Disinfection 2015;32:144-5.
- Sattar SA, Bradley C, Kibbee R, et al. Disinfectant wipes are appropriate to control microbial bioburden from surfaces: use of a new ASTM standard test protocol to demonstrate efficacy. J Hosp Infect 2015;91:319-25. [Crossref] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 26th informational supplement. M100-S26[S]. Wayne, PA: CLSI, 2016. Available online: https://clsi.org/media/1665/m100s26_correction_notice_20160110_web-1.pdf
- Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed- field gelelectrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995;33:2233-9. [Crossref] [PubMed]
- Talon D, Cailleaux V, Thouverez M, et al. Discriminatory power and usefulness of pulsed-field gel electrophoresis in epidemiological studies of pseudomonas aeruginosa. J Hosp Infect 1996;32:135-45. [Crossref] [PubMed]
- Zhu RY, Zhang XJ, Yang QW, et al. Ministry of Health National Antimicrobial Resistance Investigation Net annual report of 2011: surveillance of antimicrobial resistance in bacteria from intensive care units. The Chinese Journal of Clinical Pharmacology 2012;28:905-9.
- Qian C. Pathogen Distribution of Nosocomial Infection and Drug Resistance Analysis in Intensive Care Unit. World Latest Medicine Information 2016;16:22-23.
- Xiong XF, Li Y, Zhao JL, et al. Distribution and drug resistance of pathogens causing respiratory tract infection and cost-effectiveness analysis of antibiotics therapy. Chinese Journal of Nosocomiology 2018;28:3389-92.
- Rodrigo-Troyano A, Sibila O. The respiratory threat posed by multidrug resistant Gram-negative bacteria. Respirology 2017;22:1288-99. [Crossref] [PubMed]
- Chen L, Liu JR. Distribution and drug resistance analysis of pathogen bacteria of ICU HAI patient. Experimental and Laboratory Medicine 2017;35:926-7.
- Sun JR, Perng CL, Lin JC, et al. Ade RS combination codes differentiate the response to efflux pump inhibitors intigecycline-resistant isolates of extensively drug-resistant Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis 2014;33:2141-7. [Crossref] [PubMed]
- Wang J, Cui ZB, Wei QJ. Pathogenic Bacteria and Its Drug Resistance of Central Venous Catheter-related Bloodstream Infection in ICU. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease 2015;23:84-6.
- Lin Q, Wu SM, Li DY, et al. Effect of hand hygiene education of family members on reduction of infection rate in ICU elderly patients undergoing tracheotomy. Chinese Journal of Nosocomiology 2016;14:3354-6.
- Qin X, Wu S, Hao M, et al. The Colonization of Carbapenem-Resistant Klebsiella pneumoniae: Epidemiology, Resistance Mechanisms, and Risk Factors in Patients Admitted to Intensive Care Units in China. J Infect Dis 2020;221:S206-14. [Crossref] [PubMed]
- Chughtai AA, Seale H, Rawlinson WD, et al. Selection and Use of Respiratory Protection by Healthcare Workers to Protect from Infectious Diseases in Hospital Settings. Ann Work Expo Health 2020;64:368-77. [Crossref] [PubMed]
- Saadeh R, Khairallah K, Abozeid H, et al. Needle Stick and Sharp Injuries Among Healthcare Workers: A retrospective six-year study. Sultan Qaboos Univ Med J 2020;20:e54-62. [Crossref] [PubMed]
- Rosenthal VD, Gupta D, Rajhans P, et al. Six-year multicenter study on short-term peripheral venous catheters-related bloodstream infection rates in 204 intensive care units of 57 hospitals in 19 cities of India: International Nosocomial Infection Control Consortium (INICC) findings. Am J Infect Control 2020. [Crossref] [PubMed]
- Farag AM, Tawfick MM, Abozeed MY, et al. Microbiological profile of ventilator-associated pneumonia among intensive care unit patients in tertiary Egyptian hospitals. J Infect Dev Ctries 2020;14:153-61. [Crossref] [PubMed]


