
Complete remission in a patient with advanced renal pelvis carcinoma with lung metastasis treated with durvalumab immunotherapy: a case report
Introduction
Upper urothelial carcinomas mean that malignancies that involves the urinary tract between the calyces and the renal pelvis and the distal ureter. Renal pelvis and ureteral epithelial carcinomas comprise 95% of all upper urothelial carcinomas (1). Compared with bladder cancer, which has a high incidence, upper urothelial carcinomas are rare. In more than 50% of cases, upper urothelial carcinomas have been advanced at the time of diagnosis. Upper urothelial carcinomas can spread along the epithelium, infiltrate the renal parenchyma and the surrounding structures, and spread through the lymphatic system or blood. Usually, the tumor spreads along the epithelium from top to bottom, and precancerous lesions, such as carcinoma in situ or dysplasia, develop in the ureters. Many patients with bottom-up spread experience vesicoureteral reflux.
Total nephroureterectomy combined with bladder sleeve resection is still the gold standard for the treatment of upper urothelial carcinomas and is suitable for most patients. However, some patients still develop postoperative recurrence, especially those with advanced urothelial carcinomas. Previous studies (2,3) have shown the recurrence rate of upper urothelial carcinomas after nephrectomy and partial ureterectomy to be 30–35%
According to the POUT study (4), postoperative chemotherapy with gemcitabine plus cisplatin or carboplatin can significantly improve disease-free survival (DFS) in patients with upper urothelial carcinomas. However, while cisplatin or carboplatin-based chemotherapy can improve survival, the 5-year survival rate remains low. For more than 30 years, the standard of care in unresectable and metastatic urothelial carcinoma was cisplatin-based combination chemotherapy (5). Patients with recurrence after first-line treatment, or who show progress while receiving first-line treatment, have a particularly poor prognosis. Unfortunately, second-line chemotherapies, including paclitaxel, pemetrexed, docetaxel, and vinflunine, have shown only modest efficacy with an ORR of 12% and a median OS of 5–7 months (5-7).
In recent years, immunotherapy has brought new hope to patients with advanced urothelial carcinomas. It has been confirmed that immune checkpoint inhibitors (ICIs), such as PD-1/PD-L1 monoclonal antibodies, can achieve positive results for patients with advanced urothelial carcinomas, both as a first-line treatment and as a second-line treatment. Since 2016, there have been five ICIs (atezolizumab, pembrolizumab, nivolumab, durvalumab, and avelumab) approved by the US Food and Drug Administration (FDA) for the treatment of urothelial carcinomas (8,9), atezolizumab and pembrolizumab were approved by Korea-FDA for the treatment of metastatic urothelial carcinoma after cisplatin failure; these two drugs were also approved as the first-line treatment in patients with cisplatin-ineligible metastatic urothelial carcinoma.
Durvalumab is a selective, high-affinity, PD-L1 monoclonal antibody, which works by blocking the binding of PD-L1 with PD-1 and CD80, thus allowing T cells to recognize and kill tumor cells (10-12). In May 2017, durvalumab was approved by the FDA for the treatment of patients with urothelial carcinomas who developed to locally advanced disease or metastasis (13).
In this study, we present a case of a patient with advanced renal pelvis carcinoma, who developed lung metastasis after postoperative chemotherapy with gemcitabine combined with cisplatin at ~9 months. The patient received immunotherapy with durvalumab (620 mg IVGTT q2w, administered every 14 days), which is a human monoclonal antibody targeting PD-L1. After three cycles of treatment with durvalumab alone, the patient achieved complete remission (CR). Such cases have rarely been reported before.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1338).
Case presentation
General information
A 64-year-old male patient was admitted to our hospital in December, 2019, with lung metastases that had developed following surgery for right renal pelvis carcinoma. The patient had no fever, cough, expectoration, chest tightness, shortness of breath, back ache, hematuria, or other discomfort.
The patient had no history of type II diabetes mellitus, hypertension, hepatitis, tuberculosis, or other medical history. The patient had no smoking or drinking history, and no family history of cancer was reported.
The patient was hospitalized in the another hospital in March, 2019, after experiencing pain in the right back and acid bilge feeling for 1 month. Positron emission tomography-computed tomography (PET-CT) revealed a nodular shadow in the soft tissue of the right renal pelvis with increased fluorodeoxyglucose (FDG) metabolism; it was considered as cancer of the renal pelvis. Subsequently, the patient underwent radical surgery for right renal pelvis cancer in the another hospital on March 26, 2019. Postoperative pathology showed a cauliflower-like mass in the renal pelvis (3×2.5×1.5 cm3), which was diagnosed as high-grade urothelial papillary carcinoma with tumor thrombi in the vessels. After the operation, the patient received four cycles of chemotherapy with gemcitabine combined with cisplatin (the specific details are not known) and underwent regular reexamination was performed. In December, 2019, CT performed in the another hospital indicated multiple metastasis in both lungs. The patient was subsequently admitted to our hospital for further treatment.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Examination
Physical examination: the patient’s weight was 70kg, the patient had an Eastern Cooperative Oncology Group (ECOG) Performance Status score of 1, and a numeric rating scale (NRS) score of 0. The patient had a clear mind and was in good spirits. There was no yellow staining of the skin or sclera and no swelling of superficial lymph nodes. Double lung auscultation revealed clear breath sounds, with no dry and wet rales and a homogeneous rhythm. The abdomen was flat and soft, with old surgical scars, and no tenderness or rebound pain. The liver and spleen are not enlarged, and they are not palpable under the ribs. Neither of the lower limbs had edema. Mobility dullness (−); pathology sign (−).
Laboratory examination: the patient’s routine blood, biochemical, and tumor markers (AFP, CEA, CA199, CA125, and PSA), liver and kidney function, electrolyte levels, myocardial enzyme levels, cardiac troponin levels, B-type natriuretic peptide (BNP) levels, and thyroid function were normal.
Routine electrocardiogram (ECG): the patient had normal sinus rhythm.
Chest CT: the patient had multiple scattered solid small nodule foci in both lungs (considered as metastasis), localized emphysema in the left upper lung, slight thickening and adhesion in the left lower pleura, an elevated left diaphragm, and scattered calcification in the left lung hilum, as well as coronary atherosclerosis.
Abdomen CT and head magnetic resonance imaging (MRI): normal.
Diagnosis
Based on the findings above, the patient was diagnosed as advanced renal pelvis carcinoma with lung metastases (rt3n0m1, stage IV).
Treatment
The patient received durvalumab (commercial name: Imfinzi) every 14 days (620 mg IVGTT q2w), with the first cycle on January 14, 2020, the second cycle on January 28, 2020, and the third cycle on February 11, 2020. The treatment was not stopped until the disease progressed or the toxicity became intolerable.
During the treatment, the patient’s routine blood markers, liver and kidney function, coagulation function, thyroid function, and tumor markers were monitored weekly. After three cycles of therapy, the patient underwent chest CT examination, and the therapeutic efficacy was evaluated.
Assessment standards of therapeutic efficacy and adverse events
Therapeutic efficacy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) standard. CR is defined as the disappearance of all target lesions under CT scan or endoscopy, lasting for at least 4 weeks. Partial remission (PR) is defined as a reduction of >50% in the sum of the two longest perpendicular diameters of the target lesions, lasting for at least 4 weeks. Stable disease (SD) is defined as a ≤50% decrease or a ≤25% increase in the sum of the two longest perpendicular diameters of the target lesions, lasting for at least 4 weeks. Progressive disease (PD) is defined as a >20% increase in the sum of the two longest perpendicular diameters of at least one measureable or assessable lesion or as the appearance of a new lesion.
Adverse events were monitored and recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 3.0), which classifies adverse events into four grades (from grade I as the least severe to grade IV as the most severe). Treatment was postponed if adverse events of grade III or above occurred and was re-started with a 25% dose reduction once the adverse events had reduced to grade II. The treatment could be stopped if adverse events (grade III or above) that could not be managed even with a reduced dose occurred twice.
Evaluation of therapeutic efficacy and adverse events
The patient is still being treated with durvalumab alone to the present day. The patient showed good compliance and tolerance to the treatment. After three cycles of the treatment, a CT scan showed that the pulmonary nodules had disappeared, and no recurrence or metastasis was observed. The patient had achieved CR (Figure 1).
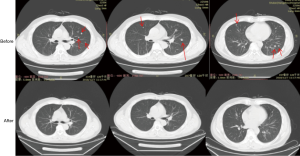
During the treatment, the patient reported slight weakness during the first few days of the third cycle of treatment, but no nausea, fever, rash, immune-related pneumonia, immune-related hepatitis, or other adverse events were observed. The patient had a ECOG score of 0.
Discussion
Upper urothelial carcinomas are highly invasive tumors which account for 5–10% of all urothelial carcinomas. Treating patients with advanced urothelial cancer presents a considerable challenge. Due to complications and renal function damage, some patients cannot tolerate platinum chemotherapy. Recent studies have confirmed the efficacy of ICIs for the treatment of various malignant tumors, including melanoma, lung, and other tumors (14-16).
Durvalumab is a PD-L1 monoclonal antibody. It functions by blocking the binding of PD-L1 with PD-1 and CD80, thus enabling T cells to recognize and kill tumor cells (10-12). Study 1108 (NCT01693562) (13) was a phase 1/2 multicenter, open-label study conducted in patients with inoperable or metastatic solid tumors. The primary end point was safety, and objective response rate (ORR, confirmed) was a key secondary end point. In the urothelial bladder cancer cohort, involving 61 patients (40 PD-L1-positive, 21 PD-L1-negative), the most common treatment-related adverse events (AEs) of any grade was fatigue (13.1%), diarrhea (9.89%), and decreased appetite (8.2%). The ORR was dependent on PD-L1 expression, 31.0% (95% CI 17.6–47.1%) in the entire cohort and 46.0% (27.5–66.1) in patients with positive PD-L1 score (≥25% expression on either tumor or immune cells). Patients with negative PD-L1 score (<25% on tumor and immune cells) did not respond to treatment with durvalumab. Consequently, durvalumab was approved by the US FDA in May 2017 for the treatment of locally advanced or metastatic urothelial carcinoma.
In this study, we reported a case of a patient with renal pelvis carcinoma who developed lung metastases during postoperative chemotherapy and went on to achieve CR after immunotherapy with durvalumab alone. In this case, the patient had received adjuvant chemotherapy of gemcitabine plus cisplatin after surgery, but relapsed 9 months postoperatively. After three cycles of treatment with Durvalumab alone, CR was reached without obvious adverse events.
Biomarkers that can help to identify the patients who can benefit from immunotherapy urgently need to be discovered and confirmed. In theory, PD-L1 expression may be a potential biomarker; however, its predictive value has yet to be confirmed, and in clinical trials, some patients with low or no PD-L1 expression have still shown a response to immunotherapy (17-19). However, in the KEYNOTE-045 study (20), the expression of PD-L1 was shown to have a certain predictive value. Moreover, microsatellite instability (MSI) is usually caused by deficient mismatch repair (dMMR), which accumulates high levels of mutations and produces new antigens, leading to increased sensitivity to PD-1/PD-L1 antibodies. This indicates that tumors with dMMR-based MSI may respond to immunotherapy.
In this case, PD-L1 and next generation sequencing were not detected before treatment. Therefore, at present, the reason why the patient showed such a good therapeutic response is not clear. Unlike PD-L1 expression and the level of tumor mutational burden (TMB) in lung cancer, which have a definite predictive value for immunotherapy, the US FDA has not yet approved the biomarkers that need to be detected to predict prognosis in ureteral cancer after immunotherapy. Some scholars also believe that MSI should be detected in patients with urothelial carcinoma. On May 24, 2017, the US FDA approved the use of pabolizumab for the treatment of solid tumors with high microsatellite instability (MSI-H)/dMMR. This is the first anti-tumor treatment not to be based on tumor source, but on biomarkers, and thus represents a milestone in precision medical treatment. In a study published in the American Journal of Surgical Pathology, Ju et al. (21) examined the expression of MMR and MSI in upper urothelial carcinomas, and compared them with those in bladder urothelial carcinomas. Tissue samples and patient information were collected from 117 and 160 cases of upper urothelial carcinomas and bladder epithelial cancer, respectively. The results showed that 10 cases (9%) of upper urothelial carcinomas were dMMR, while only 1% dMMR case was observed in bladder epithelial cancer. Among these cases, 4/10 (40%) had MSI-H, and 3/4 of dMMR had a history of colorectal cancer related to MSI-H and Lynch syndrome (LS). Combined with other studies, possibly 1–3% of cases are LS-related tumors. Therefore, the patient in this case may have had a high degree of MSI, which led to his good response to PD-L1 immunotherapy. Based on the above results, we suggest that for patients with upper urothelial carcinomas, MSI and MMR are detected. Furthermore, the predictive value of these biomarkers may not be limited to colorectal cancer and endometrial cancer, but to multiple cancer types.
Conclusions
Durvalumab proved to be an effective and safe treatment for a patient with renal pelvis carcinoma who developed lung metastases within 9 months of postoperative chemotherapy. For patients with upper urinary tract epithelial cancer, PD-L1 expression and next generation sequencing are suggested before treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1338
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1338). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yu J, Li G, Wang A, et al. Impact of squamous differentiation on intravesical recurrence and prognosis of patients with upper tract urothelial carcinoma. Ann Transl Med 2019;7:377. [Crossref] [PubMed]
- Zigeuner RE, Hutterer G, Chromecki T, et al. Bladder tumour development after urothelial carcinoma of the upper urinary tract is related to primary tumour location. BJU Int 2006;98:1181-6. [Crossref] [PubMed]
- Novara G, De Marco V, Dalpiaz O, et al. Independent predictors of contralateral metachronous upper urinary tract transitional cell carcinoma after nephroureterectomy: multi-institutional dataset from three European centers. Int J Urol 2009;16:187-91. [Crossref] [PubMed]
- Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet 2020;395:1268-77. [Crossref] [PubMed]
- Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 2017;71:462-75. [Crossref] [PubMed]
- Galsky MD, Pal SK, Lin SW, et al. Real-world effectiveness of chemotherapy in elderly patients with metastatic bladder cancer in the United States. Bladder Cancer 2018;4:227-38. [Crossref] [PubMed]
- Niegisch G, Gerullis H, Lin SW, et al. A real-world data study to evaluate treatment patterns, clinical characteristics and survival outcomes for first- and second-line treatment in locally advanced and metastatic urothelial cancer patients in Germany. J Cancer. 2018;9:1337-48. [Crossref] [PubMed]
- Donin NM, Lenis AT, Holden S, et al. Immunotherapy for the Treatment of Urothelial Carcinoma. J Urol 2017;197:14-22. [Crossref] [PubMed]
- Farina MS, Lundgren KT, Bellmunt J. Immunotherapy in Urothelial Cancer: Recent Results and Future Perspectives. Drugs 2017;77:1077-89. [Crossref] [PubMed]
- Stewart R, Morrow M, Hammond SA, et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol Res 2015;3:1052-62. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974-982. [Crossref] [PubMed]
- Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol 2016;34:3119-25. [Crossref] [PubMed]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56-61. [Crossref] [PubMed]
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-68. [Crossref] [PubMed]
- Santoni M, Montironi R, Battelli N. Immune Checkpoint Blockade in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;379:91-2. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312-22. [Crossref] [PubMed]
- Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J Clin Oncol 2017;35:2117-24. [Crossref] [PubMed]
- Bellmunt J, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015-26. [Crossref] [PubMed]
- Ju JY, Mills AM, Mahadevan MS, et al. Universal Lynch Syndrome Screening Should be Performed in All Upper Tract Urothelial Carcinomas. Am J Surg Pathol 2018;42:1549-55. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


