The role of sterile chitosan-based dressing in reducing complications related to a peripherally inserted central catheter in patients with hematological tumors
Introduction
Hematological tumors are malignant tumors originating from the hematopoietic system, among which leukemia, lymphoma, and multiple myeloma are the most common. As a special kind of tumor, the prevalence rate of hematological tumors is increasing year by year. Traditional treatment methods such as radiotherapy and chemotherapy play an important role in the fight against tumor cells, but their toxic and side effects are great and they will kill normal cells at the same time. In 2018, James and Honjo won the Nobel Prize in Physiology or Medicine for cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death receptor 1 (PD-1), respectively (1). Immune Checkpoint Blocker (ICB) controls and clears tumors by blocking abnormally activated immunosuppressive pathways, reactivating T cell immune efficacy, restoring and enhancing systemic anti-tumor immune responses, have achieved positive efficacy in many solid tumors and hematological tumors. In 2015, PD-1 inhibitor Nivolumab cured relapse/refractory classical Hodgkin's lymphoma (R/RcHL), resulting in overall response rate of 87% (2), which has inspired the researchers to wonder whether ICB would have gained good anticancer effect as a potential novel treatment.Many PD-1 and CTLA-4 blockers have been developed and put into clinical use as single drugs or combined treatment. The initial results are good and have become a new choice for patients with hematological tumors.
Hematological tumors require a long treatment time, and intravenous chemotherapy is one of the common treatments. However, peripheral intravenous chemotherapy is prone to drug leakage, vascular sclerosis, increased vascular fragility, vascular stenosis, and reduced vascular elasticity, making it difficult or impossible to infuse any solution (3). A peripherally inserted central catheter (PICC) can address this problem. During the procedure, a catheter is inserted via a peripheral access (basilic vein, median cubital vein, cephalic vein) and advanced into the superior vena cava. PICC placement is widely used for chemotherapy in patients with hematological tumors. However, PICC sheaths cause greater vascular damage due to their large size, which, along with weakened immunity and coagulation disorders in patients with hematological tumors, increases the risks of bleeding, infection, phlebitis, and poor healing at the puncture point after PICC placement. This affects treatment, prolongs hospitalization, and increases medical expenses if not treated properly (4). Chitosan is a naturally occurring biocompatible polysaccharide that can activate the coagulation pathway and form an isolation layer to protect the wound. It reduces and stops bleeding, prevents infection, and promotes wound healing and is thus widely used to dress wounds after surgery (5). Seethamsetty found that Chitosan dressing is a competent hemostatic agent that significantly reduced the post-extraction bleeding, with better pain control and lower incidence of wound infection (6). To date, no studies have investigated its role after PICC placement. In this study, we analyzed the clinical data of patients with sterile chitosan-based dressing after PICC placement between April 2018 and March 2020 and found that sterile chitosan-based dressing significantly reduced PICC-related complications. Interestingly, the situation of extravasation within 24 hours, infection, and healing were used as dependent variables to conduct ordered multi-classification logistic regression analysis, and multiple variables were found to be correlated with these factors. The results are reported below. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1235).
Methods
General information
We retrospectively analyzed the clinical data of 216 patients with hematological tumors who received treatment via a PICC at the Department of Hematology, Maoming People’s Hospital, between April 2018 and March 2020. Among them, 205 patients met the entry criteria and were included in this study. Inclusion criteria were as follows: (I) patients who were eligible for PICC placement; (II) patients who were conscious and had the ability to cooperate with PICC placement; and (III) patients who received catheter maintenance or follow-up by a trained nurse for at least a week after PICC placement. Exclusion criteria were as follows: (I) critically ill patients who were unconscious or unable to cooperate; and (II) patients with persistent bleeding at the puncture site during or after PICC placement. The patients were divided into two groups, the control group (sterile gauze dressing; n=100) and the observation group (sterile chitosan-based dressing; n=105). This study was approved by the Medical Ethics Committee of Maoming People’s Hospital (ID: PJKY2020 MI-001-01). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients.
Procedure
A 4-Fr single-lumen catheter with a three-way valve (C. R. Bard, Inc., USA) was inserted with the modified Seldinger technique under the guidance of ultrasound B and maintained by a trained PICC specialist nurse of our department. In the control group, common gauze dressing was used to compress and secure the PICC: 2.5 cm × 1.5 cm 24-layer gauze (made from 10 cm × 10 cm nonwoven fabric) was used to compress the puncture site, which was then covered with 12 cm × 15 cm self-adhesive film dressing (SOFIT 3000) and another layer of 2.5 cm × 1.5 cm 24-layer gauze (made from 10 cm × 10 cm nonwoven fabric) and secured with 1 cm × 8 cm elastic tape. In the observation group, 6 cm × 7 cm sterile chitosan-based dressing (Figure 1) was used to cover the puncture site, which was then covered with 2.5 cm × 1.5 cm 24-layer gauze (made from 10 cm × 10 cm nonwoven fabric), 12 cm × 15 cm self-adhesive film dressing (SOFIT 3000), and another layer of 2.5 cm × 1.5 cm 24-layer gauze (made from 10 cm × 10 cm nonwoven fabric), and secured with 1 cm × 8 cm elastic tape. In both groups, the patients were instructed to press the puncture site with their thumb for at least 30 minutes. Accurate platelet counts are done by SK8800 Automatic blood cell analyzer using an electronic particle counting method. Accurate platelet counts are done by SK8800 Automatic blood cell analyzer using an electronic particle counting method. The patients received PICC-related health education once tip position was confirmed by X-ray. Moreover, the patients were instructed to minimize limb movement on the puncture side, not raise their arms for 24 hours, minimize strenuous limb movement on the puncture side for 3 days, and not lift any heavy object while the PICC was in place.
Evaluation methods
A trained attending nurse changed the dressings for PICCs. Bleeding at the puncture site was recorded during the first dressing change, and infection and healing at the puncture site were recorded at 1 week. During the first dressing change, bleeding at the puncture site was rated as “0” if there was no blood or minimal dark blood that did not exceed one-quarter of the area of the gauze or penetrate layer 2 of the lower layer of the sterile gauze, “1” if dark blood covered one-quarter to three-quarters of the area of the gauze or penetrated layer 2 or 3, “2” if dark blood covered the entire gauze or penetrated layer 4, and “3” if fresh blood was discovered on the gauze or bleeding was observed at the puncture site (7). For infection at the puncture site, no abnormal sign at the puncture site indicated no infection, redness and swelling indicated mild infection, firmness indicated moderate infection, and the presence of pus indicated severe infection (8). For puncture site healing, adherence of skin to the catheter (the catheter could not easily move) at the puncture site indicated good healing, and no adherence of skin to the catheter (easy catheter movement) indicated poor healing (4). Phlebitis was evaluated according to the Infusion Therapy Standards of Practice from the Infusion Nurses Society [2006]: 0: no symptoms; I: redness at the infusion site with or without pain: II: pain at the infusion site with redness and/or swelling; III: pain at the infusion site with redness and/or swelling and palpable cord-like veins; IV: pain at the infusion site with redness and/or swelling, palpable cord-like veins (>2.5 cm or 1 inch), and pus (9).
Statistical analysis
SPSS v20.0 was used for data analysis. Normally distributed measurement data are expressed as mean ± standard deviation and were analyzed with the independent-sample t-test. Count data are expressed as frequency (percentage) and were analyzed with the chi-squared test. Multivariate analysis was performed with logistic regression. P<0.05 was considered statistically significant.
Results
Overall medical complications
Table 1 shows the general information of the patients, and Table 2 shows the details of PICC placement. Table 3 shows the incidences of PICC-related complications. The overall incidences of bleeding, infection, and poor healing were, respectively, 42.9%, 6.7%, and 6.7% in the observation group and 63.0%, 22.0%, and 34% in the control group (all P<0.05). Among the 205 patients, the overall incidences of catheter displacement, phlebitis, and thrombosis were 3.9%, 2.4%, and 2.4%, respectively, with no significant between-group difference.
Full table

Full table

Full table
Multivariable analyses of the incidence of local bleeding
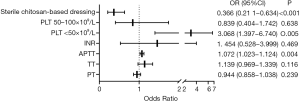
The patients were divided into three grades by platelet count (<50×109/L, 50×109–100×109/L, >100×109/L). Pearson’s correlation coefficient was calculated to analyze the correlations between the dependent variable, bleeding within 24 hours of PICC placement, and the independent variables, which were sterile chitosan-based dressing, sex, age, body mass index (BMI), primary disease, hypertension, diabetes mellitus, initial placement, number of puncture attempts, incision methods, smooth placement, placement site, venous access, tip position, platelet subgroups, prothrombin time (PT), thrombin time (TT), activated partial thromboplastin time (APTT), and international normalized ratio (INR). Variables with P<0.1 were taken into the next step. Sterile chitosan-based dressing, platelet subgroups, PT, TT, APTT, and INR were incorporated into the ordinal multinomial logistic regression equation as independent variables. The results showed that the risk of local bleeding in the sterile chitosan-based dressing group was 0.366 times that in the common dressing group, and the risk of local bleeding in the platelets <50×109/L group was 3.068 times that in the platelets >100×109/L group (P<0.05), while the risk of local bleeding was not significantly different between the platelets 50×109–100×109/L and >100×109/L groups (P>0.05). See Figure 2.
Change in complications using sterile chitosan-based dressing
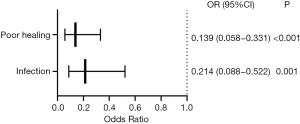
Pearson’s correlation coefficient was used to analyze the correlations between the dependent variables, infection and healing, and independent variables, including sterile chitosan-based dressing, sex, age, BMI, primary disease, hypertension, diabetes mellitus, initial placement, number of puncture attempts, incision methods, smooth placement, blood loss during placement, placement site, venous access, tip position, catheter displacement, PLT, PT, TT, APTT, and INR to identify significant variables at P<0.1. Sterile chitosan-based dressing was the only variable that was incorporated into the logistic regression equation. The results showed that the risk of infection and the risk of poor healing in the sterile chitosan-based dressing group were, respectively, 0.214 times and 0.139 times those in the common dressing group (both P<0.05). See Figure 3.
Discussion
Reliable venous access is essential for infusion therapy, especially in patients with hematological tumors. Intravenous infusion and catheter maintenance are important parts of nursing care. Infusion therapy is often administered via a peripheral catheter, but the catheter must be replaced regularly to prevent catheter-related complications, such as bleeding, phlebitis, and thrombophlebitis. A central catheter provides reliable venous access without repeated venipuncture, but it may cause life-threatening complications, such as pneumothorax, hemothorax, and bleeding (10).
Since the 1980s, PICC has been widely used to establish smooth vascular access. In addition to playing a key role in the treatment of patients with critical and potential life-threatening conditions, it has become an important tool for the treatment of patients with hematological tumors and provides the central venous access for long-term administration of antibiotics and chemotherapy drugs. It is easy and convenient to insert, causes few complications, provides reliable venous access, and has high patient satisfaction (11,12). Nevertheless, PICC-related complications have been reported, such as catheter-related bloodstream infection (CRBSI), deep vein thrombosis, and bleeding at the puncture site.
After PICC placement, bleeding at the puncture site is common in patients with hematological tumors. Some patients have low platelet count, a risk factor for bleeding at the puncture site. Bleeding may also occur in patients with a low level of coagulation factors. Some studies have shown that cancer-related thrombocytopenia increases the risk of bleeding, with a negative correlation between bleeding rate and platelet count, especially in patients with hematological malignancies (13). Moreover, for patients with hematological tumors, thrombocytopenia significantly increases the risk of bleeding, especially when platelets are <50×109/L, as increased capillary fragility increases the bleeding rate (14,15). This study showed similar results: the risk of bleeding in the platelets <50×109/L group was 3.068 times that in the platelets >100×109/L group. In addition, chemotherapy drugs may irritate and damage the vascular mucosa, resulting in increased vascular permeability and bleeding at the puncture site. To address this problem, Wan et al. used alginate dressing that released natural celluloses, polysaccharides, and carbohydrates to promote wound healing. Upon contact with the wound exudate, the alginate dressing released calcium ions to accelerate clotting, thereby effectively reducing bleeding at the PICC puncture site (16). In this study, we used sterile chitosan-based dressing or sterile gauze to cover the PICC puncture site and found that sterile chitosan-based dressing had a superior hemostatic effect (local bleeding, P<0.05). In addition, the risk of local bleeding in the sterile chitosan-based dressing group was 0.366 times that in the common dressing group (Figure 1), which was consistent with the findings of Seethamsetty et al. (6). Chitosan is composed of N-acetylglucosamine and glucosamine. It is widely used in the medical field because of its superior biological properties, such as cell compatibility and anti-pyrogenic activity. Chitosan is a naturally occurring biocompatible polysaccharide that can activate the coagulation pathway to promote hemostasis. Due to its positive charge, chitosan attracts negatively charged red blood cells and platelets to form a sealed isolation film at the wound, which is further enhanced as more platelets and red blood cells accumulate at the wound to promote hemostasis and wound healing (17).
CRBSI is closely related to immunity. In patients with hematological tumors, PICC placement significantly increases the risk of (severe) infection due to neutropenia. Some studies have shown that infection was the most common and severe complication of PICC, with 1.5 to 6.6 infections per 100 catheter days. Bertoglio et al. conducted a prospective study and found that the incidence of CRBSI was 4.0% per catheter day (18). Fairhall showed that the incidence of PICC-related CRBSI was 4.3% per catheter day (19). Chitosan has unique antimicrobial activity and non-antigenicity, and its antimicrobial activity has been extensively studied. Conventional dressings often encapsulate antimicrobial agents, which are gradually released to achieve antimicrobial effects. In contrast, chitosan-based dressings have intrinsic, sustained antimicrobial activity against a wide range of microbial strains and are less toxic to tissues and cells (17,20). Similarly, this study showed that the incidence of infection was significantly lower in the sterile chitosan-based dressing group than in the common sterile dressing group (OR 0.214; Figure 2).
Sterile chitosan-based dressing was also superior to the common sterile dressing in promoting wound healing, (93.33% vs. 66%, P<0.05). Chitosan attracts inflammatory cells to the wound via its chemotactic effect, activates macrophages to synthesize and secrete interleukin 8, stimulates neovascularization and accelerates granulation tissue formation at the wound, and reduces the high level of carbonic acid to indirectly improves the oxygen level at the wound, thereby promoting wound healing and repair (21).
Phlebitis is another common PICC-related complication. Its main causes include intimal damage due to repeated catheter movement in the vessel during or after placement and a local inflammatory response due to drug irritation and subsequent release of inflammatory mediators (22). The incidence of phlebitis is approximately 1.2% to 54.5% (23). In this study, the incidence of phlebitis was only 2.4%, which was attributable to careful operation by our nurses, proper securing and maintenance of the PICC, and patient education. Unclean dressing and postoperative infection are also risk factors for phlebitis (24). In this study, the incidence of infection was significantly lower in the observation group than in the control group, but no significant between-group difference was observed in the incidence of phlebitis, which may be related to the small sample size.
Conclusions
For patients with hematological tumors, sterile chitosan-based dressing effectively reduces the incidence of PICC-related adverse events such as bleeding and infection and promotes wound healing. This is a small study, and larger studies are needed to validate its results.
Acknowledgments
Funding: This work was supported by the High-level Hospital Construction Research Project of Maoming People’s Hospital [Reference No. Yueweihan (2018) 413].
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1235
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1235
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1235). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of Maoming People’s Hospital (ID: PJKY2020 MI-001-01). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zang X. 2018 Nobel Prize in medicine awarded to cancer immunotherapy: Immune checkpoint blockade - A personal account. Genes Dis 2018;5:302-3. [Crossref] [PubMed]
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311-9. [Crossref] [PubMed]
- Johansson E, Hammarskjöld F, Lundberg D, et al. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol 2013;52:886-92. [Crossref] [PubMed]
- Duwadi S, Zhao Q, Budal BS. Peripherally inserted central catheters in critically ill patients - complications and its prevention: A review. Int J Nurs Sci 2018;6:99-105. [Crossref] [PubMed]
- Moeini A, Pedram P, Makvandi P, et al. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr Polym 2020;233:115839. [Crossref] [PubMed]
- Seethamsetty S, Sarepally G, Sanober A, et al. A Comparative Evaluation of the Effectiveness of Chitosan-Based Dressing and Conventional Method of Hemostasis in Patients on Oral Antithrombotic Therapy without Therapy Interruption. J Pharm Bioallied Sci 2019;11:S18-S23. [Crossref] [PubMed]
- Min C, Jie Z, Ying L, et al. Study on first dressing change time after PICC placement among patients with hematological malignancy. Journal of Nursing Science 2019;34:44-6.
- Gorski LA. The 2016 Infusion Therapy Standards of Practice. Home healthcare now 2017;35:10-8. [Crossref] [PubMed]
- Infusion Nurses Society. Infusion Nursing Standards of Practice. J Infus Nurs 2006;29:S1-S92. [PubMed]
- Woller SC, Stevens SM, Evans RS. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative: A summary and review of peripherally inserted central catheter and venous catheter appropriate use. J Hosp Med 2016;11:306-10. [Crossref] [PubMed]
- Baxi SM, Shuman EK, Scipione CA, et al. Impact of postplacement adjustment of peripherally inserted central catheters on the risk of bloodstream infection and venous thrombus formation. Infect Control Hosp Epidemiol 2013;34:785-92. [Crossref] [PubMed]
- Cotogni P, Barbero C, Garrino C, et al. Peripherally inserted central catheters in non-hospitalized cancer patients: 5-year results of a prospective study. Support Care Cancer 2015;23:403-9. [Crossref] [PubMed]
- Gaydos LA, Freireich EJ, Mantel N. The quantitative relation between platelet count and hemorrhage in patients with acute leukemia. N Engl J Med 1962;266:905-9. [Crossref] [PubMed]
- Larsen JB, Hojbjerg JA, Hvas AM. The Role of Platelets in Cancer-Related Bleeding Risk: A Systematic Review. Semin Thromb Hemost 2020;46:328-41. [Crossref] [PubMed]
- Mithoowani S, Cervi A, Shah N, et al. Management of major bleeds in patients with immune thrombocytopenia. J Thromb Haemost 2020;18:1783-90. [Crossref] [PubMed]
- Wan G, Yan M. Puncture Point Hemostatic Effect Observation of Different Materials with Modified Seldinger Technique in PICC Catheter. Zhongguo Yi Liao Qi Xie Za Zhi 2015;39:388-90. [PubMed]
- Radwan-Pragłowska J, Piątkowski M, Deineka V, et al. Chitosan-Based Bioactive Hemostatic Agents with Antibacterial Properties-Synthesis and Characterization. Molecules 2019;24:2629. [Crossref] [PubMed]
- Bertoglio S, Faccini B, Lalli L, et al. Peripherally inserted central catheters (PICCs) in cancer patients under chemotherapy: A prospective study on the incidence of complications and overall failures. J Surg Oncol 2016;113:708-14. [Crossref] [PubMed]
- Fairhall M. An Observational Study of Peripherally Inserted Central Cather (PICC)-Related Complications Amongst Oncology Patients. 2008.
- Hu H, Xu FJ. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater Sci 2020;8:2084-101. [Crossref] [PubMed]
- Du L, Tong L, Jin Y, et al. A multifunctional in situ-forming hydrogel for wound healing. Wound Repair Regen 2012;20:904-10. [Crossref] [PubMed]
- Urbanetto JS, Muniz FOM, Silva RMD, et al. Incidence of phlebitis and post-infusion phlebitis in hospitalised adults. Rev Gaucha Enferm 2017;38:e58793. [PubMed]
- Braga LM, Parreira PM, Oliveira ASS, et al. Phlebitis and infiltration: vascular trauma associated with the peripheral venous catheter. Rev Lat Am Enfermagem 2018;26:e3002. [Crossref] [PubMed]
- Miliani K, Taravella R, Thillard D, et al. Peripheral Venous Catheter-Related Adverse Events: Evaluation from a Multicentre Epidemiological Study in France (the CATHEVAL Project). PLoS One 2017;12:e0168637. [Crossref] [PubMed]