
Diagnosis of complex regional pain syndrome type 1 in patients with corticobasal degeneration: a case report
Introduction
Complex regional pain syndrome type I (CRPS I) is characterized by a continuing pain that is disproportionate in degree or time to the typical course of any trauma or other lesions (1). The pain is regional and not in a specific nerve territory, and often involves abnormal motor, sensory, vasomotor, sudomotor, and/or trophic findings (1). This syndrome shows variable progression over time, and involves motor symptoms, including weakness, bradykinesia, dystonia, myoclonus, and tremor that could occur in the later phase of CRPS I (1,2). Proper diagnosis and management of CRPS I is important because these CRPS I symptoms can lower the quality of life and activities of daily living (ADL) of its patients (3).
Corticobasal degeneration (CBD) is a neurodegenerative disorder that clinically presents as progressive asymmetric motor symptoms that show no response to levodopa. The symptoms include dystonia, bradykinesia, rigidity, and myoclonus as well as symptoms of cortical and basal ganglia dysfunction, including alien limb phenomenon, asymmetric apraxia, and cortical sensory loss (4,5). CBD and CRPS I can mimic each other because of their similar motor symptoms, especially in the chronic phase of CRPS I (6). Only one case study has reported a CBD patient who was initially misdiagnosed with CRPS I because of their similar clinical findings; however, there has been no reported case of a patient being diagnosed with both CBD and CRPS I (6).
In this case report, we present a CBD patient who was also diagnosed with CRPS I. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-918).
Case presentation
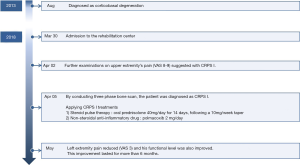
A 76-year-old man patient who had been diagnosed with CBD was admitted to our rehabilitation center on March 30, 2018. He did not have any past stoke history, similar family history, or any previously performed surgical procedure. His medical records indicated that he had been diagnosed with CBD several years prior by a specialist on movement disorder at the department of neurology of our hospital and that he could walk with minimal assistance at the time. However, as his functional level worsened with time, he could not gait at all and showed severe asymmetry, postural instability, limb rigidity, limb dystonia, tremor, ideomotor apraxia, and bradykinesia especially on his left upper extremity on admission at our rehabilitation center. Entire timeline of the patient from diagnosis to treatment is shown in Figure 1.
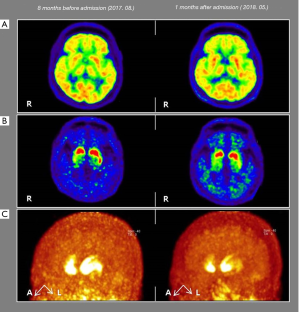
Brain magnetic resonance imaging showed no other remarkable findings except for diffuse brain atrophy. 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) scan images were acquired 30 minutes after intravenous injection of 18F-FDG PET (311 MBq) by collecting emission PET data for 10 minutes in 3-dimensional mode after brain CT. They showed a mild to moderate decrease in metabolism in the bilateral parietotemporal lobes and a slight and asymmetric decrease in metabolism in the right frontoparietal lobes (Figure 2A). 18F-florinated-N-3-fluoropropyl-2-β-carboxymethoxy-3-β-(4-lodophenyl) nortropane (18F-FP-CIT) PET scanning was also conducted 8 months before admission and 1 month after admission to assess the integrity of the patient’s nigrostriatal dopaminergic system (Figure 2B,C). Image acquisition started 2 hours after intravenous injection of 18F-FP-CIT PET (195 MBq), and emission PET data were collected for 10 minutes in three-dimensional mode after brain CT. 18F-FP-CIT PET imaging revealed an asymmetrically decreased FP-CIT uptake in the bilateral posterior putamen (Right > Left) with relative sparing of the ventral putamen, which showed an aggravated status compared to the previous scan conducted 8 months before admission. Moreover, although he was on anti-parkinsonism medications, including a total levodopa dose of 1550 mg/day and a rasagiline dose of 1 mg/day, he did not show sustained responsiveness to levodopa medication.
The mini-mental state examination score of the patient was 25, which was attributed to errors made in orientation to time, writing, and drawing of two pentagons. Motor tests showed that his left upper and lower extremities had decreased Medical Research Council (MRC) scores compared to his right side; specifically, the manual muscle test (MMT) scores for the shoulder flexors, elbow flexors and extensors, wrist extensors, and finger flexors and abductors were 4/1 while those for the hip flexors, knee extensors, ankle dorsiflexors and plantar flexors, and long toe extensors were 4/3 (7) (Table 1). He also showed severe rigidity; specifically, his left elbow was overextended and pronated and the wrist and finger was over flexed, which was similar to the previous alien limb phenomenon that also showed complex unintentional limb movements that interfered with normal tasks.

Full table
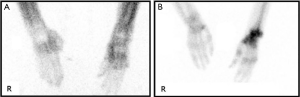
The severe asymmetrical tremor, limb rigidity, and dystonia observed in his left upper arm corresponded to his known underlying CBD; however, the patient also presented unexpected severe left upper extremity pain with a visual analogue scale (VAS) score of between 8 and 9 on admission. Given this severe pain, the examiner hardly performed the physical examination, including the motor and passive range of motion (ROM) tests. The patient also could neither transfer well nor lie on his left side, and he woke up from his sleep more than 10 times during the night due to severe pain. Therefore, we decided to evaluate the possible reasons for the left upper extremity pain. At first, we conducted radiographic scans of the left shoulder, elbow, wrist, and fingers to rule out any muscular skeletal problem. The radiographs only showed osteopenia of the left upper extremity but no significant bone fracture and joint problems, such as joint space narrowing, which can cause the pain. For further evaluation, additional history taking and physical examination were performed, and the left extremity of the patient showed darkened skin color change, edema, reduced skin elasticity, cold skin temperature, wet skin, and limited ROM compared to the right side, which are suggestive of CRPS I. Therefore, an additional three phase bone scan was conducted that revealed an increase of blood flow, pool, and delayed periarticular uptake in the left wrist and hand as well as relatively increased bone and joint uptake in the left upper extremity, which led us to diagnosis the patient with typical CRPS (Figure 3). Based on this diagnosis, the patient was started on steroid pulse therapy with oral prednisolone 40 mg/day for 14 days, following a 10 mg/week taper (2). We also simultaneously administered Polmacoxib 2 mg/day (a non-steroidal anti-inflammatory drug; NSAID) to reduce the pain (2). Following this treatment, his left extremity pain reduced from a VAS score of 8–9 to 3. The MMT score of the shoulder flexors also improved from 1 to 3 while that of the elbow flexors and extensors, wrist extensors, and finger flexors and abductors improved from 1 to 3+ (Table 1). He was also able to release and grasp an object clumsily using his left upper extremity because he could move his upper extremity against gravity (MRC, 3-3+). The rigidity and limited ROM of his left upper extremity were also improved, and he could transfer and lie on his left side easily. In addition, severe pain did not wake him up from his sleep during the night. This improvement in symptoms lasted for more than 6 months. To share the rare two diseases’ co-existence with other physicians, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
In this case report, we present for the first time a CBD patient who suffered from severe pain and was also diagnosed with CRPS I. Administration of proper treatment for CRPS I, including steroid pulse therapy and NSAIDs, not only reduced the pain but also improved the patient’s quality of life and ADLs.
CRPS I and CBD share similar features; specifically, the clinical symptoms of CBD, including weakness, bradykinesia, dystonia, myoclonus, tremor, and pain can also be presented in the chronic stage of CRPS I (6). Only one case study in 2009 reported a patient who met the criteria of CBD, and who was initially thought to have CRPS I with clinical symptoms of severe paroxysmal pain, temperature changes, skin edema and discoloration, abnormal sudomotor function, dystonia, stiffness, jerking tremor, and postural instability (6). In this previously reported patient, motor symptoms, balance, postural instability, ideomotor apraxia, mood disturbance, cognitive impairment, confusion, and visual hallucinations worsened with time and he was finally diagnosed with CBD according to Riley and Lang’s criteria six years after symptom onset (6,8). They also compared the clinical features of CRPS I and CBD and found that dystonia occurred in 91% and 71% of CRPS I and CBD cases, respectively, whereas pain was observed in most CRPS I cases but only in 3% of CBD cases (6,9). Therefore, they suggested that CRPS I could be classified among the CBD-like syndromes, such as progressive supranuclear palsy, Creutzfeldt-Jakob disease, frontotemporal dementia, Alzheimer’s disease, parkinsonism linked to chromosome 17, and multi-infarct disorder (6,9,10). In our case report, the patient had been priory diagnosed with CBD and suffered from severe pain on his left arm. Initially, we thought that CBD was responsible for the pain because 3% of CBD patients also experience pain (6). However, based on his clinical history, physical examination, and imaging studies, such as three phase bone scan, we finally diagnosed him with CBD accompanied by CRPS I. In addition, administration of proper treatment for CRPS I improved his upper extremity pain, quality of life, and ADLs.
In conclusion, we for the first time present a CBD patient who also suffered from CRPS I. We suggest asserted that clinicians be aware of the differential diagnosis of CRPS I from CBD because they could have similar clinical features. Moreover, proper management based on a precise diagnosis is important because these symptoms can affect the patients’ quality of life and ADLs. In addition, it can be assumed that the mutual involvement of the cortex, basal ganglia, and thalamus attributes to the similarity in clinical features (10,11). To our knowledge, this is the first report of a CBD patient being also diagnosed with CRPS I. However, several limitations should be considered. First, our current case report is based on a single case. However, given the low estimated prevalence of both diseases, it is hard to find a patient with a simultaneous diagnosis of both CBD and CRPS I (12). Second, we observed limited functional improvements in the patient, which can be attributed to his severely poor functional level on admission due to the progression state of CBD. Thus, further large studies considering these limitations are warranted.
Acknowledgments
Funding: This study was supported by a Veterans Health Service Medical Center Research Grant, Republic of Korea (grant number: VHSMC19014).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-918
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-918). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harden NR, Bruehl S, Perez RS, et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for complex regional pain syndrome. Pain 2010;150:268-74. [Crossref] [PubMed]
- Schott GD. Complex? Regional? Pain? Syndrome? Pract Neurol 2007;7:145-57. [Crossref] [PubMed]
- Rome L. The place of occupational therapy in rehabilitation strategies of complex regional pain syndrome: comparative study of 60 cases. Hand Surg Rehabil 2016;35:355-62. [Crossref] [PubMed]
- Wadia PM, Lang AE. The many faces of corticobasal degeneration. Parkinsonism Relat Disord 2007;13:S336-40. [Crossref] [PubMed]
- Mahapatra RK, Edwards MJ, Schott JM, et al. Corticobasal degeneration. Lancet Neurol 2004;3:736-43. [Crossref] [PubMed]
- Gatto EM, Garretto NS, Etcheverry JL, et al. Corticobasal degeneration presenting as complex regional pain syndrome. Mov Disord 2009;24:947-8. [Crossref] [PubMed]
- Bove G. Medical Research Council. Aids to examination of the peripheral nervous system: Memorandum no. 45. London: Her Majesty’s Stationary Office, 1976.
- Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord 2003;18:467-86. [Crossref] [PubMed]
- Johnsen JA, Miller VT. Tobacco intolerance in multiple system atrophy. Neurology 1986;36:986-8. [Crossref] [PubMed]
- Spillane JD. The effect of nicotine on spinocerebellar ataxia. Br Med J 1955;2:1345-51. [Crossref] [PubMed]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 2006;27:482-91. [Crossref] [PubMed]
- de Mos M, de Bruijn AG, Huygen FJ, et al. The incidence of complex regional pain syndrome: a population-based study. Pain 2007;129:12-20. [Crossref] [PubMed]


