
Effects of exogenous probiotics on the gut microbiota and clinical outcomes in critically ill patients: a randomized controlled trial
Introduction
Gut possesses immunoregulatory function, which is dependent on the microbiota, intestinal barrier and intestinal immune system. The gut microbiota is a complicated ecosystem that consists of a large amount of microorganisms, participating in the growth, nutrition metabolism and aging of the host. During the course of critical illness and the following medical interventions, the composition and phenotype of intestinal microorganisms experience significant changes, leaving the host susceptible to opportunistic infection and even leading to System Inflammatory Reaction Syndrome (SIRS) or Multiple Organ Dysfunction Syndrome (MODS). In short, the disturbance of microbiota leads to the “undrained abscess” which increases complications and causes poor prognosis (1,2).
Probiotics are live microorganisms which may have a health benefit on the host when adequate amount of probiotics is administered (3). They can inhibit the potential pathogenic micrograms (PPMs) and help maintain the stability of gut microbiota through enhancement of barrier function, immunomodulatory function, and secretion of bacteriocin (4). It has been shown that probiotics are promising to maintain the balance of gut microbiota and may serve as an alternative therapy to gastrointestinal diseases (1). Furthermore, the use of probiotics is also reasonable in critical illness patients, such as patients receiving major abdominal surgery, traumatic patients and intensive care unit (ICU) patients, since the available findings about probiotics seem to be encouraging (5-8). In the critically ill patients who are at high risk of disturbance of gut microbiota and immunosuppression, the benefit of probiotics remains inconclusive. This study was conducted to investigate the effects of probiotics on the gut microbiota, intestinal barrier and clinical outcomes in critically ill patients. The authors have completed the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/apm-20-202).
Methods
Patients and setting
The study was conducted in a teaching school affiliated to Fudan University in China, and patients were recruited from the respiratory intensive care unit (RICU). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Ethics Committee of the hospital (No. 2012055) and informed consent was taken from all the patients. This study was registered in Clinical Trial Management Public Platform (No. 12002854).
Patients newly admitted to RICU were included in the study, and the exclusion criteria were as follows: (I) patients were younger than 18 years; (II) patients had an Apache II score less than 10 points; (III) patients had an explosion to microecological preparations in the past 2 months; (IV) patients had a history of disease that has the potential to affect the gut microbiota such as gastrointestinal cancer, short intestinal syndrome and end-stage hepatic cirrhosis.
Once the informed consent was obtained, patients were randomized to probiotic group or control group. The assignment was done on admission. Clinicians who were responsible for the data collection and analysis were blind to the grouping.
Probiotic treatment
The probiotic agent, MIYA-BM® tablets (Miyarisan pharmaceutical Co., Ltd., Tokyo, Japan), contains Clostridium butyricum at 106 CFU bacteria per sachet. One tablet of MY or a placebo tablet was administered thrice daily. If oral intake was infeasible, probiotic tablet was dissolved in 50–100 mL of sterile water and given via a nasogastric/orogastric tube. Patients received routine treatments in the RICU.
Data collection and endpoints
The endpoints were as follows: the patient was discharged, mortality, rate of hospital-acquired infection, hospital stay and medical cost, cost for antibiotics, time of antibiotics treatment. The vital signs, gastrointestinal symptoms, abdominal manifestations, body temperature fluctuation, and interventional measurements were also monitored in each patient.
Samples collection and microbiota detection
The samples were collected on the day of assignment and at two weeks after admission. The stools and serum were transferred for detection within 30 min; the DNA was extracted according to the manufacturer’s instruction and stored at −20 °C. Then, real-time PCR was performed to quantify the overwhelming microbiota of gastrointestinal tract, including Bacteroides, Escherichia coli, Enterococcus, Bifidobacterium and Lactobacillus. The PCR system and primers are shown in Table 1. The standard control was confirmed by sequencing. The primers were designed after screening at the GenBank to meet the sensitivity and specificity.

Full table
The serum contents of diamine oxidase (DAO), lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10) after administration of probiotics were detected by ELISA.
Statistical analysis
Data were stored on a Microsoft Excel spreadsheet, and statistical analysis was performed using STATA for Windows Version 10.0 (SPSS Inc., Chicago, IL, USA) (StataCorp. Texas, USA). Qualitative data were compared using the two-tailed Chi-square test. Quantitative data with normal distribution are expressed as means ± standard deviation (SD), while data with abnormal distribution as medians and interquartile range. Comparisons of quantitative data were done with analysis of variance (ANOVA) or paired t-tests. The Mann-Whitney U-test was used to compare the nonparametric data. A value of P<0.05 was considered statistically significant. Univariate and logistic regression models were used for correlation variables.
Results
Characteristics of patients
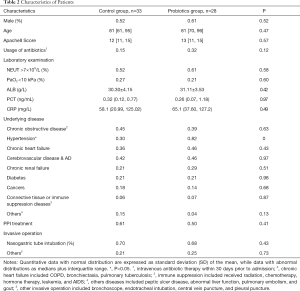
Sixty-two eligible patients were recruited from August 2013 to March 2014, of whom 61 completed the study (Figure 1). The demographics and baseline characteristics were comparable between two groups. In addition, most of the risk factors of Ventilator Associated Pneumonia (VAP) such as invasive interventions were also similar between two groups (Table 2). The acute exacerbation of chronic obstructive pulmonary disease was the leading cause of hospitalization in these patients. The diseases responsible for the hospitalization included chronic obstructive disease (42.62%), chronic heart failure (40.98%), cerebrovascular disease (44.26%), and diabetes (21.31%); 8.2% of patients were intubated and 68.85% of patients fed via a nasogastric tube.
Full table
Primary endpoints and clinical manifestations
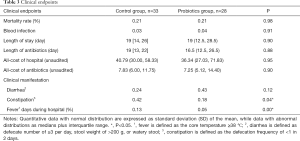
There was no significant difference in the mortality between probiotics group and control group (21.21% vs. 21.43%, P=0.98). The risk factors of death included exposure to antibiotic in past month before admission (OR =6.83, P=0.01), duration of fever during hospitalization (OR =1.12, P=0.03), LPS level on admission (OR =0.98, P=0.06) and use of nasogastric tube (OR =1.88, P=0.08). In addition, the independent risk factors of death included antibiotic prescription (adjusted OR =11.91, P=0.07) and fever days (adjusted OR =1.29, P=0.09) (Table 3).
Full table
Most of patients had infection, mainly pneumonia, on admission, and thus it was hard to distinguish therapeutic failure from hospital acquired pneumonia once fever, deterioration of lung symptoms and presence of lung consolidation were observed in the RICU. This was same to diarrhea we cannot tell the reason is infection or just because of lake of nutrition if it happened 48 h after in charge. Therefore, the fever and diarrhea after 48 h of admission were excluded hospital acquired infection was confirmed in 4 patients by microbiological examination. There were 3 patients in the control group: blood infection (cerebrovascular disease) in 1 patient, urinary infection in 1 patient and pneumonia in 1 patient. There was 1 patient with blood infection (cryptococcus) in the probiotics group. There was no significant difference in the nosocomial infection between two groups. The hospital stay, duration of antibiotics treatment, medical cost and cost for antibiotics were also comparable between two groups (Table 3).
The incidence of diarrhea and constipation was 63.93% (39/61), and there was no marked difference between two groups (66.67% vs. 60.71%, P=0.63). Probiotics treatment significantly reduced the incidence of constipation (17.86% vs. 42.42%, P=0.04). The duration of day (%) during hospitalization in the probiotic group was significantly lower than in the control group (4.85% vs. 12.94%, P=0.00).
Effect of probiotics on gut microbiota
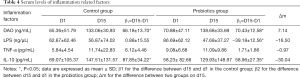
In order to investigate the interaction between gut microbiota and clinical prognosis, the gut microbiota, inflammatory factor and endotoxin were detected in these patients. β1 and β2 presented the dynamic changes of the bacterial quantity in controlled and probiotic group, respectively; Δm displayed the difference of bacterial quantity between probiotics group and control group on day 15. Positive Δm indicated an increase after 2 weeks, while negative Δm indicated a decrease (Table 4).
Full table
After 2-week antibiotics treatment, the amount of Bacteroides remained unchanged in both probiotics group and control group (β1=0.20, P=0.50; β2=−0.21, P=0.25). The amount of Escherichia coli tended to reduce in the probiotics group and control group (β1=−0.04, P=0.88; β2=−0.58, P=0.15). The amount of Enterococcus showed a reduced tendency in the probiotic group (β2=−0.32, P=0.16) while an increased tendency was noted in the control group (β1=0.44, P=0.28). In addition, the amounts of Bifidobacterium and Lactobacillus also remained unchanged after treatment in two groups.
Bacteroides yielded a significantly decrease after probiotics treatment (Δm=−0.69, P=0.048), while Escherichia coli and Enterococcus showed decreased tendencies in the probiotics group (Δm=−0.65, P=0.08; Δm=−0.52, P=0.22) as compared to control group. The amounts of Bifidobacterium and Lactobacillus were comparable between two groups.
The content of DAO, an indicator of intestinal epithelial barrier, significantly elevated in both control group and probiotics group (β1=66.18, P<0.01; β2=70.43, P<0.01), but probiotics treatment had no influence on the content of DAO. The serum LPS level significantly decreased in the probiotics group (β2=−39.18, P=0.002) while it remained relatively stable in the control group. There was a significant increase in the serum IL-10 level in both groups, but it was similar between two groups (β1=87.85, P=0.01; β2=58.96, P=0.03). The serum TNF-α level remained unchanged in two groups, and there was no marked difference between two groups.
Discussion
Effect of gut microbiota on human health and disorder
Probiotics are involved in regulation of immunity system, nutrition metabolism, regulate response to stress and other processes of the host (4). Probiotics help to keep the composition of intestinal flora stablely, which is of great importance to host. Immune tolerance and immune defense are important for the balance of microbiota: immune tolerance can distinguish colonized microbiota from pathogens while immune defense can stimulate protective defensive response to prevent excessive inflammatory damage.
Probiotics differ in species and action pattern and can activate the immune system mainly via microorganism-associated molecular patterns (MAMPs) and pattern recognition receptors (PRRs). MAMPs refers to flagellin, secretory protein and LPS. CD4+ T lymphocyte proliferate once stimulated by polysaccharide A (PSA) of germ-free Clostridium difficile. The differentiation of T cell towards Th2 and Treg T cells is induced after stimulation by lactobacillus (9). In addition, Bacteroides induce immune tolerance through PPAR pathway (10). On the other hand, the pathogenicity of LPS is largely reduced under the effect of intestinal alkaline phosphatase (LAP) induced by G- bacteria and physical isolation.
Modulating the gastrointestinal microbiota through the use of probiotics is a safe and well-tolerated approach (11). Since gut microbiota helps modulate nutrient metabolism, immune system and bacteriostasis and maintain microflora stability and physical health, physiological disorders and diseases will develop once the gastrointestinal microflora imbalance is present. It has been proven that probiotics help reduce the community acquired upper respiratory infection in children (12) and delay the colonization of Pseudomonas aeruginosa in the respiratory tract of mechanically ventilated patients (7). On the other hand, probiotics harbor relative stable genetic materials that unlikely incorporate exogenous resistance gene or horizontal transmit.
Above all, probiotics surpasses the traditional antibiotics not only in safety and immune modulation, but also in the reduced risk of inducing bacterial resistance and supra-infection. It is alternative for some natural drug resistant pathogens. Increasing studies focus on the mechanism and application of promising probiotics.
Effect of probiotics on the clinical outcomes in critically ill patients
Studies have demonstrated that probiotics can attenuate the malnutrition and inflammation in the elderly (13), and reduce the risk of hospital acquired infection in severe trauma patients (5,14,15). It seems to be promising that the probiotics can effectively prevent the occurrence of ventilator associated pneumonia (16). However, it remains controversial on the application of probiotics in the critically ill patients. Our study failed to prove the protective effects of probiotics in the critically ill patients because there were no significant differences in the mortality, blood infection and hospital stay between probiotics group and control group.
The risk factors for bacterial translocation include the destroy of mucosal barrier and the proliferation of potentially pathogenic microorganisms. Those who underwent abdominal surgery are more likely to suffer from the intestinal ischemia. Hemorrhagic shock dramatically destroys the barrier function, resulting in bacterial translocation which may be attenuated by probiotics (17). Another common cause of bacterial translocation is chemotherapy. The administration of cyclophosphamide with enema may directly destroy the tight conjunction in the intestinal barrier and dramatically increase the PPMs (18). For patients underwent invasive interventions such as mechanical ventilation and gastric catheter indwelling, the bacterial translocation has a high incidence. It has been shown that the pathogens isolated from the respiratory tract are the same clones to those from gastrointestinal tract, suggesting the gastrointestinal microbiota as a source of pathogens responsible for indigenous infection. As far as we are concerned, patients tend to experience acute infection on admission, followed by gastrointestinal dysfunction. It not only added to the disturbance of intestinal flora but also eliminated the local probiotic concentration and protective function when iatrogenic measures were adopted such as prescription of antibiotics, change of feeding ways and intestinal motility. MIYA-BM® tablets are acid tolerant and seldom affected by antibiotics. It had reported that Clostridium can be detected after Clostridium butyricum administration in the gastric or duodenal ulcers patients, but none was positive for clostridium after 2-week Helicobacter pylori eradication treatment (19). However, the Clostridium was not found in the probiotics group on the 15th day, we speculate that probiotics may be affected in critically ill patients compared with health people. It has been reported that intestinal ischemia, abnormal intestinal motility and use of antibiotics can affect the gastrointestinal flora. However, the unbalance of microbiota seems to turn back to the “setting point” of normal condition after transient change (20). The setting point is determined based on the heritage, immune status, environment and diet. Exogenous supplementation of probiotics is hard to have a long-term effect, and the protective function will terminate once the supplementation is discontinued (20). On the other hand, antibiotics exerts profound and direct impact by inducing resistance PPMs on the epithelial cells, resulting in recurrent and refractory infection.
Effect of probiotics on clinical manifestations
The disturbance of microbiota flora may cause a series of gastrointestinal symptoms such as constipation, diarrhea and abdominal distension. It has been reported that the incidence of bowel obstruction in the ICU is 40% and it is mainly caused by an overgrowth of colon bacteria in the proximal intestine (21). The disturbance of microbiota flora is one of risk factors for bacterial translocation and highly related to the bacterial load when the bacterial translocation is present (22). In addition, the disturbance of microbiota flora may cause the generation of a great amount of metabolic products which are harmful for nutritional state and may aggravate the tissue injury (23). Our results showed MY significantly reduced the occurrence of constipation which may be related to relief of bacterial burden and reduction of toxin produced by pathogenic bacteria.
Influence of probiotics on the microbiota flora in critically ill patients
The anaerobes decrease to 100–10,000 times especially for Bifidobacterium, Lactobacillus while staphylococcus increase to 100 times in the critically ill patients as compared to healthy controls (1). It has been reported that, in the critically ill patients, prophylactic synbiotics may have preventive effects on the enteritis and VAP (24). After enteral feeding with food containing Lactobacillus for bed-ridden elderly in-patients, the fecal microbiota remained stable at any time point between groups except for an increased tendency of lactobacillus in the intervention group (13). A mixture of bifidobacterium and lactobacillus elevated the concentration of bifidobacterium in the mechanically ventilated patients, and decreased the levels of P. aeruginosa, Enterococcus, and Enterobacteria depending on the increased concentration of organic acid (25). Above all, lots of studies were cross-section studies. So, we conducted a prospective follow-up study to investigate the microbiota flora in critically ill patients. We intended to demonstrate the relationship between the dynamic change of microbiota flora and clinical outcomes. In our study, our results suggested that probiotics have the potential to decrease the amount of Bacteroides, E. coil and Enterococcus in the critically ill patients although there was no significant difference between two groups. This indicates probiotics fail to improve the primary clinical outcomes in the critically ill patients.
Different probiotics are varied in their ability to resist gastric acid and bile acids, colonize the intestinal tract, and resist to pathogens. Bacteroide are the overwhelming bacteria in the intestine and colon and play an important role in the polysaccharides metabolism. B. fragilis strains are opportunistic pathogens and the leading anaerobic isolates in the clinical specimens, which lead disease by LPS and endotoxin and always resist to β-lactam antibiotic (26). Enterococci coli have been recognized as the widely prevalent hospital-acquired pathogens can disseminate drug resistance gene (27). Enterococci coli are also the major type of bacteria responsible for bacterial translocation in animal models. Probiotic bifidobacteria can help protect mice from infection with Shiga toxin-producing Escherichia coli O157: H7, MRSA, duovirus, flu virus and other pathogens (28,29). Nowadays, we always focused on the studies about Lactobacillus, Bifidobacterium, Clostridium butyricum. However, the microbia is so complicated that it seems long before fully undisclosed.
Gut microbiota including probiotics are inevitably influenced by iatrogenic measures. The iatrogenic measures increase the risk of PPMs colonization in the gastrointestinal tract (30). The microbiota will be suppressed soon after the use of antibiotics, and the type, half-life, route of administration, and pharmacological characteristics of antibiotics are related to its influence on the gut microbiota (4). Physicians prefer for advanced, broad-spectrum antibiotics in critically ill patients. Our results showed there was a decreased tendency in the amount of Bifidobacterium after probiotics treatment as compared to controls on admission. The balance of gut microbiota should be taken into consideration when clinical decision is made, and unnecessary parenteral nutrition and excessive anti-acid treatment should be avoided (31).
The gastrointestinal tract is composed of the epithelium, mucous, submucosa and muscularlayer Bacteroides, Bifidobacterium, Streptococcus, and Enterobacteriaceae are dominant at the submucosa, while the Lactobacillus and Enterococcus are rich in the mucous layer. So fecal samples are inconclusive exhibition of gut microbiota and gradually substituted by mucosa biopsy. The genetic, environment, age, diet and antibiotic exposure all contribute to the composition of gut microbiota, and thus there is significant diversity between individuals. Consequently, it is likely to obtain a negative result in a population.
Immunomodulation and inflammatory regulation
DAO is an endoenzyme with high activity and expressed in all mammal intestinal mucosa, specifically in the jejunum and ileum. DAO may enter the intercellular space, lymphatic vessel and blood in case of gastrointestinal diseases, and thus DAO has been used as an indicator of intestinal injury and loss of mucosal integrity (32). Our results showed probiotics had no protective effect on the intestinal mucosa after 2-week treatment. LPS is mainly responsible for the sepsis and other pathophysiological changes such as septic shock/MODS caused by Gram negative bacteria, and LPS and LBP have the potential to predict blood infection (32). Our results indicated there was a significant decrease in the serum LPS of the probiotics group, which was not found in the control group. We speculate that probiotics help attenuate the invasion of LPS in critically ill patients. As aforementioned, it is possible that there is another method for inactivating LPS apart from the intestinal barrier or physical separation (33). The decrease of E. coli after probiotics treatment indicated that probiotics reduce the LPS by lowering Gm-bacteria load in the gut. Besides, probiotics are capable of enhancing the intestinal barrier which reduces the translocation of LPS from the intestine. The duration of fever was significantly reduced, which may be related to the reduction of serum LPS.
In addition, our results showed serum IL-10 significantly elevated, but there were no significant differences in the serum IL-10 and TNF-α levels between control group and probiotics group. B. bifidum, L. lactis and L. acidophilus are the potent inducers of IL-10 and inhibitor of TNF-α, IL-2 and IL-6 (34,35). Cascade response of massive inflammatory factors in initiate SIRS and compensated anti-inflammatory response syndrome (CARS) is spontaneously activated, presenting with elevation of inhibiting inflammatory factor such as IL-10 and damage to immune defense which predicts the risk of death and recurrent infection (36). Various clinical conditions and therapeutic means increase the complexity and delicacy of the joint of SIRS and CARS system. But it lacks efficacy for the variation of IL-10, TNF-α to distinguish the function of probiotics. TNF-α releases at the acute phase of infection and the short half-time requires continuous monitor. It remains great challenge for the study of inflammatory state in critically ill patients.
Conclusions
Above all, probiotics have limited influence on the gut microbiota. Our study fails to show the beneficial effects of probiotics on the primary clinical outcomes in critically ill patients. In addition, the probiotics have no effect on the impaired intestinal barrier although the serum LPS concentration reduces after probiotics treatment, indicating that probiotics help reduce the burden of Gm-bacteria from the gut. The relationship between the host and the gastrointestinal microbiota is complicated and more studies are needed to confirm our findings in the future.
Acknowledgments
Funding: This work was supported by the National Basic Research Program (973 Program) in China (2013CB531402).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-202
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-202
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-202). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Ethics Committee of the hospital (No. 2012055) and informed consent was taken from all the patients. This study was registered in Clinical Trial Management Public Platform (No. 12002854).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shimizu K, Ogura H, Asahara T, et al. Probiotic/synbiotic therapy for treating critically ill patients from a gut microbiota perspective. Dig Dis Sci 2013;58:23-32. [Crossref] [PubMed]
- Montejo JC. Enteral nutrition-related gastrointestinal complications in critically ill patients: a multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit Care Med 1999;27:1447-53. [Crossref] [PubMed]
- Heselmans M, Reid G, Akkermans LM, et al. Gut flora in health and disease: potential role of probiotics. Curr Issues Intest Microbiol 2005;6:1-7. [PubMed]
- Sekirov I, Russell SL, Antunes LC, et al. Gut microbiota in health and disease. Physiol Rev 2010;90:859-904. [Crossref] [PubMed]
- Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med 2010;182:1058-64. [Crossref] [PubMed]
- Giamarellos-Bourboulis EJ, Bengmark S, Kanellakopoulou K, et al. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma 2009;67:815-21. [Crossref] [PubMed]
- Forestier C, Guelon D, Cluytens V, et al. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care 2008;12:R69. [Crossref] [PubMed]
- Knight DJ, Gardiner D, Banks A, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med 2009;35:854-61. [Crossref] [PubMed]
- Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology 2008;123:197-208. [PubMed]
- Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol 2004;5:104-12. [Crossref] [PubMed]
- Manuzak JA, Hensley-McBain T, Zevin AS, et al. Enhancement of Microbiota in Healthy Macaques Results in Beneficial Modulation of Mucosal and Systemic Immune Function. J Immunol 2016;196:2401-9. [Crossref] [PubMed]
- Hatakka K, Savilahti E, Ponka A, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. Bmj 2001;322:1327. [Crossref] [PubMed]
- Fukushima Y, Miyaguchi S, Yamano T, et al. Improvement of nutritional status and incidence of infection in hospitalised, enterally fed elderly by feeding of fermented milk containing probiotic Lactobacillus johnsonii La1 (NCC533). Br J Nutr 2007;98:969-77. [Crossref] [PubMed]
- Spindler-Vesel A, Bengmark S, Vovk I, et al. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr 2007;31:119-26. [Crossref] [PubMed]
- Rayes N, Seehofer D, Theruvath T, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation--a randomized, double-blind trial. Am J Transplant 2005;5:125-30. [Crossref] [PubMed]
- Gu WJ, Deng T, Gong YZ, et al. The effects of probiotics in early enteral nutrition on the outcomes of trauma: a meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr 2013;37:310-7. [Crossref] [PubMed]
- Luyer MD, Buurman WA, Hadfoune M, et al. Strain-specific effects of probiotics on gut barrier integrity following hemorrhagic shock. Infect Immun 2005;73:3686-92. [Crossref] [PubMed]
- Yang J, Liu KX, Qu JM, et al. The changes induced by cyclophosphamide in intestinal barrier and microflora in mice. Eur J Pharmacol 2013;714:120-4. [Crossref] [PubMed]
- Shimbo I, Yamaguchi T, Odaka T, et al. Effect of Clostridium butyricum on fecal flora in Helicobacter pylori eradication therapy. World J Gastroenterol 2005;11:7520-4. [Crossref] [PubMed]
- Barman M, Unold D, Shifley K, et al. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun 2008;76:907-15. [Crossref] [PubMed]
- Malbrain ML, Chiumello D, Pelosi P, et al. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med 2004;30:822-9. [Crossref] [PubMed]
- Van Felius ID, Akkermans LM, Bosscha K, et al. Interdigestive small bowel motility and duodenal bacterial overgrowth in experimental acute pancreatitis. Neurogastroenterol Motil 2003;15:267-76. [Crossref] [PubMed]
- Simenhoff ML, Dunn SR, Zollner GP, et al. Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Miner Electrolyte Metab 1996;22:92-6. [PubMed]
- Shimizu K, Yamada T, Ogura H, et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: a randomized controlled trial. Crit Care 2018;22:239. [Crossref] [PubMed]
- Hayakawa M, Asahara T, Ishitani T, et al. Synbiotic therapy reduces the pathological gram-negative rods caused by an increased acetic acid concentration in the gut. Dig Dis Sci 2012;57:2642-9. [Crossref] [PubMed]
- Sears CL. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin Microbiol Rev 2009;22:349-69. Table of Contents. [Crossref] [PubMed]
- Werner G, Coque TM, Franz CM, et al. Antibiotic resistant enterococci-tales of a drug resistance gene trafficker. Int J Med Microbiol 2013;303:360-79. [Crossref] [PubMed]
- Asahara T, Shimizu K, Nomoto K, et al. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect Immun 2004;72:2240-7. [Crossref] [PubMed]
- Gluck U, Gebbers JO. Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and beta-hemolytic streptococci). Am J Clin Nutr 2003;77:517-20. [Crossref] [PubMed]
- Niederman MS, Craven DE. Devising strategies for preventing nosocomial pneumonia--should we ignore the stomach? Clin Infect Dis 1997;24:320-3. [Crossref] [PubMed]
- Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 2001;1:101-14. [Crossref] [PubMed]
- Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol 2003;3:169-76. [Crossref] [PubMed]
- Bates JM, Akerlund J, Mittge E, et al. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2007;2:371-82. [Crossref] [PubMed]
- Timmerman HM, Niers LE, Ridwan BU, et al. Design of a multispecies probiotic mixture to prevent infectious complications in critically ill patients. Clin Nutr 2007;26:450-9. [Crossref] [PubMed]
- Kyo M, Nishioka K, Nakaya T, et al. Unique patterns of lower respiratory tract microbiota are associated with inflammation and hospital mortality in acute respiratory distress syndrome. Respir Res 2019;20:246. [Crossref] [PubMed]
- Muszynski J, Nateri J, Nicol K, et al. Immunosuppressive effects of red blood cells on monocytes are related to both storage time and storage solution. Transfusion 2012;52:794-802. [Crossref] [PubMed]



