
Invasive angiomyxoma diagnosed by transvaginal ultrasound: a case report
Introduction
Aggressive angiomyxoma (AAM) is a clinically rare benign mesenchymal soft tissue tumor. This type of tumor is non-enveloped and colloid, and it is located in the rich mucinous stroma. AAM is characterized by invasion and recurrence, which was first proposed by Steeper (1) in 1983 and named “aggressive angiomyxoma”. AAM is common in women and occurs frequently in women of childbearing age (2). AAM occurs frequently in the perineum, pelvic cavity, and vulva, and the duration of onset varies from several months to more than 10 years. There are few reports on CT and MR imaging findings of hemangiomas (3,4). This article further describes and summarizes the ultrasound manifestations of aggressive hemangioma in this case. We can improve the detection rate of AAM through differential diagnosis with other diseases in ultrasonic examination. We present the following case in accordance with the CARE Reporting Checklist (available at http://dx.doi.org/10.21037/apm-20-321).
Case presentation
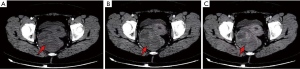
The patient, female, 54 years old, was admitted to our hospital for “pain in her right hip with mass for 3 years” on May 30, 2019. The patient reported no family history and psychosocial history, including related genetic information. Three years ago, the patient had no obvious cause of a mass and pain on the right hip. As the mass gradually increased, the bilateral hips were asymmetrical. The pain was obvious when the mass was enlarged, and it could radiate to the right lower abdomen and the right waist. The patient did not manage the lump within three years, only taking pain medication when she was in pain. Only to achieve the purpose of relieving the patient’s pain symptoms. On May 30, 2019 A dynamic enhanced pelvic CT scan report is described as that (Figure 1): medium to low mixed density is seen on the right side of the rectum, and it extends down to the right perineum. The capsule and partition are visible, and the capsule and partition are significantly strengthened. The boundary between the adjacent pelvic muscles and the rectum is unclear, and the rectum is displaced by compression. Hints: pelvic tumorous lesions are highly likely. Transvaginal ultrasound examination report is described as that on May 30, 2019 (Figure 2): heterogeneous hypoechoic masses behind the uterus to the right of the pelvis, about 18.0 cm × 4.3 cm × 9.0 cm, originates from the lower part of the posterior wall of the vagina and grows in the posterior direction of the uterus, with clear boundaries and soft texture. It presents gelatinous, easily deformed when squeezed, and most of the internal echoes are irregular. Liquid dark areas are locally visible, and it shows poor sound transmission. CDFI: rich blood flow signals within the clumps is explored, which can detect the arterial blood flow spectrum, RI: 0.64. Hints: the hypoechoic mass on the right side of the pelvis is recommended for ultrasound guided biopsy.
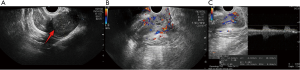
A transvaginal ultrasound guided pelvic mass biopsy was performed on June 1, 2019. The pathological result shows: (pelvic mass) there are a few proliferating spindle cells with slightly plump nuclei, loose and swollen cellular interstitium, and more blood vessels, which may not exclude the possibility of AAM.
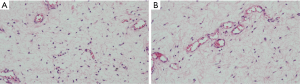
Patients underwent routine blood tests and chest X-ray examinations before surgery, and there were no other abnormalities. The patient underwent “pelvic mass resection and partial coccygeal resection” on June 8, 2019. During the operation, the cystic mass was explored and it was adhered to the surrounding tissues. The pathological results of the pelvic mass after surgery are shown (Figure 3): (pelvic mass) mesenchymal tumor. The tumor is composed of well-differentiated spindle-shaped stellate cells with interstitial mucus degeneration, and vascular proliferation can be seen, which is consistent with deep AAM (size 12 cm × 10 cm × 7 cm). Immunohistochemical results shows: CD34 (vascular +), Vimentin (+), Desmin (-), SMA (-), ERG (vascular +), Ki-67 (+) about 1%, S100 (-). There are no diagnostic challenges. Six months after the operation, the blood examination and ultrasound examination were normal. So far, less than one year after the operation, no recurrence tendency or adverse events were found. In addition. The patient’s medical history is shown in timeline in Figure 4.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
The onset of AAM is insidious, with no symptoms at an early stage, and growth is slow. It usually performs as a local mass, and a few patients may present urinary tract irritation symptoms or swelling of the lower abdomen. Although AAM is a benign tumor, due to its invasive nature, the scope of the invasion is not clear during surgery, and it is not completely resected. The residual lesions easily cause recurrence after surgery. The pathogenesis of the disease has not yet been cleared, but Kenny Moynihan (5) believes that the occurrence of the disease is related to chromosome loss.
Ultrasound is used as an early screening method for pelvic lesions. According to the existing article reports combined with this case (6), common ultrasound manifestations of AAM are: (I) due to rich mucus interstitial background, the tumour often performs low or mixed echo, irregular shapes, and borders are unclear. It often infiltrates into the surrounding soft tissue space in the form of “finger-shaped” or “tongue-shaped”; (II) characteristically is “vortex-shaped”. Mostly due to the collagen fibrous tissue pulling the surrounding mucinous stroma, the solid components within the lesion are iso-echo to hyper-echo “layered” or “swirl-like”. Sometimes the division can be seen; (III) most AAM’s CDFI shows that the abundant inside and around blood flow signal can be detected. (IV) Cystic non-echoic area is seen in the lesion area, which may be related to tumor necrosis and liquefaction. (V) Elastography shows that the texture of the lesion is soft.
The disease needs to be differentially diagnosed with myxoid liposarcoma, angiomyofibroblastoma (AMF), Bartholin’s gland cyst, and intermuscular myxoma: (I) myxoid liposarcoma originates from primitive mesenchymal cells, and ultrasound often shows low echo masses, uneven internal echo, clear boundary, and no obvious blood flow signal in CDFI; (II) the origin of AMF and AAM are all related to myofibroblasts, so the histological characteristics are similar, and there is no significant difference in imaging examinations. But AMF occurs in young and middle-aged women, AMF often occurs in the vulva, more often in the labia majora, but a few cases occur in male perineum, scrotum and other places. Unlike AAM, the lesions of AMF are generally smaller, and It is not easy to relapse after resection; (III) Bartholin’s gland cyst’s ultrasound manifests as cystic masses, even distribution of internal echo, transparent sound in the cyst is acceptable, smooth cyst wall and no blood flow signal. Generally, AAM can be misdiagnosed as Bartholin’s gland cyst, so CDFI was used to initially distinguish between Bartholin’s gland cyst and AAM. (IV) Ultrasound of intermuscular myxoma shows low-echo clumps between muscle groups, regular morphology, clear borders, and in homogenous internal echoes. Most of cases shows posterior enhanced echo. It performs “bright ring” sign, hyperechoic cyclic structure surrounded around agglomerates. AAM also needs to be distinguished from myxoid malignant fibrous histiocytoma and cellular angiofibroma by pathological diagnosis.
Pathological examination is the gold standard for diagnosing AAM: tumors are mostly non-enveloped or less enveloped, and more small blood vessels are seen inside. Tumor cells are star-shaped or short spindle-shaped on the mucus background, and infiltrating smooth muscle cells and inflammatory cells are seen around the mass. Tumor cells are eosinophilic, and the nucleus is slightly deep-dyeable. CT images often show a slightly lower density mass and CT enhancement image are mildly enhanced. MRI T1WI usually shows uneven low-signal shadows and T2WI shows non-uniform high-signal shadows (higher than muscle tissue). CT and MR images can show specific “lamellar” and “swirl” changes in the tumor. In immunohistochemistry (7), CD34, Vimentin, Desmin, SMA, and ER are often positively expressed, while CEA, CK, and S100 are often negative. Because AAM is a benign tumor, for the treatment of AAM, the surgical method is generally selected, but because of its invasive growth, follow-up and long-term observation are required after surgery.
The advantage of this case: the patient’s tumor site is special, close to the rectum, and is deep. We summarized this case from the perspective of ultrasound. The limitation of this case: although we made a preliminary diagnosis of AAM from two-dimensional ultrasound and tumor blood flow, no new shear wave elastography technology was used to examine the tumor. The patient’s opinion after treatment is that measures should be taken as soon as symptoms appear. The patient’s self-reported state is very good, and surgery is a reliable treatment.
Conclusions
AAM is a rare clinical benign tumor. Because of the aggressiveness and recurrence rate of the disease, combined with the age, location, and imaging-specific “vortex-like” appearance of the disease, AAM can be early diagnosed. The summary of AAM ultrasound imaging performance can make clinical doctors have a more intuitive understanding and understanding of this case. Through ultrasound examination of patients, it can attract the attention of clinical doctors and improve the early detection rate of AAM. Pathology is the gold standard for AAM, but early screening of AAM by ultrasound is feasible.
Acknowledgments
We are thankful to the department of Pathology, the Affiliated Hospital of Qingdao University, who provided the confirmation of the histologic diagnosis of the patient. We are thankful to Miss Fu from the department of radiology, the Affiliated Hospital of Qingdao University, who provided the confirmation of the radiology diagnosis of the patient.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE Reporting Checklist. Available at http://dx.doi.org/10.21037/apm-20-321
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-321). The authors have no conflicts of interest to declare.
Ethical Statement: Authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Published reports and pictures have been obtained from patients with informed consent. And we guarantee the true validity of the case.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Steeper TA, Rosai J. Aggressive angiomyxoma of the pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft tissue neoplasm. Am J Surg Pathol 1983;7:463-75. [Crossref] [PubMed]
- Dierickx I, Deraedt K, Poppe W, et al. Aggressive angiomyxoma of the vulva: a case report and review of literature. Arch Gynecol Obstet 2008;277:483-7. [Crossref] [PubMed]
- Benson JC, Gilles S, Sanghvi T, et al. Aggressive angiomyxoma: case report and review of the literature. Radiol Case Rep 2016;11:332-5. [Crossref] [PubMed]
- Oh S, Sung DJ, Sim KC, et al. A rare case of vulvar angiomyofibroblastoma: MRI findings and literature review. J Obstet Gynaecol 2017;37:831-3. [Crossref] [PubMed]
- Kenny Moynihan MB, Hagen J, Mcintosh DJ, et al. Loss of an X chromosome in aggressive angiomyxoma of female soft parts: a case report J. Cancer Genet Cytogenet 1996;89:61-4. [Crossref] [PubMed]
- Zhao CY, Su N, Jiang YX, et al. Application of ultrasound in aggressive angiomyxoma: Eight case reports and review of literature. World J Clin Cas 2018;6(14).
- Chan YM, Hon E, Ngai SW, et al. Aggressive angiomyxoma in females: is radical resection the only option. Acta Obstet Gynecol Scand 2000;79:216-20. [Crossref] [PubMed]



