
Current usage status of somatostatin and its analogs and trypsin inhibitors: a real-world study of 34,654 Chinese adult patients with acute pancreatitis
Introduction
Acute pancreatitis (AP) refers to auto-digestion of the pancreas, where pancreatic enzymes injure the pancreatic tissue, leading to dysfunction of the gland, remote organs, and systems. Globally, the annual incidence of AP is 34 per 100,000 people (1) and continues to increase yearly. From 2006 to 2014, its annual incidence increased by 18%. AP is currently one of the most common gastrointestinal disorders that cause hospitalization in the US and costs the health care system $9.3 billion annually. To add, ~30,000 inpatients are reported in the US every year, with 2.64 billion USD of medical expenses being paid every year (2-4). According to the International 2012 Revised Atlanta Classification for Acute Pancreatitis, AP is mainly classified as mild (MAP), moderate (MSAP), and severe (SAP) (5). Common etiologies include cholelithiasis (including biliary microlithiasis), hypertriglyceridemia, and alcohol consumption. In China, biliary pancreatitis is the primary etiology of AP (6). Currently, supportive treatment is usually employed as initial treatment for AP patients and includes fluid resuscitation, pain control, nutrition support, and maintenance of organ function.
For the use of AP drugs that inhibit exocrine secretion by the pancreas and inhibit trypsin, somatostatin and its analogs (SSTA) and trypsin inhibitors (TSI) are currently mainly used in clinical practice. SSTA (somatostatin, octreotide) can directly inhibit exocrine secretion by the pancreas while TSI drugs (ulinastatin, gabexate) can broadly inhibit the secretion and activity of trypsin, elastase, phospholipase A, and other hydrolases that are associated with AP progression. TSI can also stabilize lysosome membranes, improve pancreatic microcirculation, and reduce the occurrence of AP complications.
China has a large population and a high number of AP patients. Therefore, physicians have ample experience in the use of drugs to treat AP. SSTA and TSI drugs are recognized by many physicians for the clinical treatment of AP, and China’s authoritative guidelines and consensus have recommended these two treatment methods (6,7). The results of previous studies showed that TSI drugs can effectively reduce the mortality of AP patients (8,9). However, most of the selected studies are retrospective cohort studies and case-control studies, and there is a lack of high-quality multicenter randomized controlled trials to further validate these drug classes. Epidemiological data and studies on the current status of drug treatment of AP are outdated. The age of onset of AP, comorbidities, clinical pathway, drug treatment protocol, and hospitalization costs are intimately associated with patient outcomes and financial burden. Because of the aforementioned reasons, Changhai Hospital attempted to collect real-world study data on AP diagnosis and treatment from many hospitals in China to carry out mining and analysis of the disease characteristics of AP patients in China and real-world utilization status of SSTA and TSI drugs to provide a real-world basis and reference for rational drug administration for AP by clinicians.
We present the following article in accordance with the STROBE Reporting Checklist (available at http://dx.doi.org/10.21037/apm-19-363).
Methods
Study design
We conducted a retrospective study base on a large medical database in Changhai Hospital, Shanghai. A cohort of all patients admitted to the hospitals in this database, from January 1, 2015 to June 30, 2017, was investigated.
Aim of the study
Herein, we aimed to examine the current usage status of SSTA and TSI in Chinese adult AP patients.
Data source
Data were obtained from the research service platform database of Changhai Hospital which has the electronic medical records of 15 million inpatients every year. These patients were from 192 general hospitals in China. Data that are accessible for every patient included demographic data (age, sex, medical insurance), hospital characteristics (number of beds, region, grade, and teaching status), discharge diagnosis [primary diagnosis and other diagnosis, including name of diagnosis and international classification of diseases 10th version (ICD-10th) code], dosing record (name of drug, administration method and dose, and time), payment records (payment details and payment time), and records of surgeries and relevant tests and examinations. The main aim of constructing the database is to monitor drug resistance in the regional medical system. Previous studies on community-acquired pneumonia provided detailed descriptions and explanations of this database (10).
The study was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). All patients were anonymous and specific information that could be used for patient identification was not included, such as identification numbers and address. The review and approval of this study was exempted by the ethics committee of Changhai Hospital.
Study population and classification
We identified patients aged ≥18 with a primary discharge diagnosis of AP (ICD-10th: K85) from January 1, 2015 to June 30, 2017. To exclude the effects of daytime ward on the study, patients with a hospitalization length less than 3 days were excluded from the study.
The 2012 Revised Atlanta Classification for Acute Pancreatitis classified AP according to severity: mild AP (MAP, absence of organ failure and local or systemic complications), moderate AP (MSAP, transient organ failure or presence of local or systemic complications), and severe AP (SAP, persistent organ failure). However, not all electronic medical records in China state the severity of AP in the discharge diagnosis for every patient. Therefore, we examined the discharge diagnosis for every patient and defined MAP as the absence of text description on local or systemic complications, organ failure, and moderate and severe pancreatitis in the discharge diagnosis and no record of intensive care unit transfer in the diagnosis process. The diagnosis description for local and systemic complications includes acute peri-pancreatic fluid collection, acute necrotic collection, walled-off necrosis, pancreatic pseudocyst, organ failure, sepsis, systemic inflammatory response syndrome, shock, pancreatic encephalopathy, disseminated intravascular coagulation, ketoacidosis, abdominal compartment syndrome, and hypocalcemia. In the remaining non-MAP patients, we defined SAP as patients who fulfilled any of the following criteria: serum creatinine >170 µmol/L and persistence for more than 48 h, persistent mechanical ventilation for more than 48 h, and persistent usage of vasopressors for more than 48 h (Figure 1).

Study endpoints
The main focus of this study was to explore the application of SSTA and TSI drugs that are approved for marketing in China for AP treatment. These drugs include somatostatin, octreotide, gabexate, and ulinastatin. Drug use-related markers include the utilization rate of drugs, number of days of drug usage, time of first dose (time from first use of drug to admission), and drug costs. We used the defined daily dose (DDD) for horizontal comparison of dosage between different drugs (11). Drug DDD values were mainly obtained from searching the World Health Organization website. If no drug-related information was available on the website, the package insert was used to determine daily dose. Finally, the DDD values for somatostatin, octreotide, gabexate, and ulinastatin were found to be 6, 0.7, 300 mg, and 300,000 units, respectively. The drug dose used during the period of time divided by the DDD of the drug is used to obtain the summary DDD (DDDs) for the period. Usually, DDDs represent the frequency of drug usage. During evaluation of the overall drug prescription volume, we used DDDs/1,000 patients/day (i.e., mean consumption of a drug every day in 1,000 patients). In addition, we defined drug utilization index (DUI) as the DDDs of a time period divided by the actual number of days of drug administration to measure the daily dose for each drug use. A DUI equal to 1 indicates that the actual usage of the drug conforms to the DDD recommended by World Health Organization.
Statistical analysis
Descriptive analysis was mainly used for data statistics. Data distribution was expressed as mean and 95% confidence interval (CI). If data were skewed, they were expressed as median and upper and lower quartiles. First, we described the baseline characteristics of all patients and baseline characteristics after stratification by severity (including age, sex, chronic comorbidity score, and hospital characteristics such as number of beds). The Charlson Comorbidity Index was used to evaluate chronic comorbidities which were converted to ICD-10th codes. Thereafter, drug usage status and treatment methods of somatostatin, octreotide, gabexate, and ulinastatin in patients with different disease severities (MAP, MSAP, and SAP) were examined. To add, drug usage trends in every quarter and prescription trends of all patients during the 30-day admission from 2015 to 2017 were evaluated. DUI and DDDs/1,000 patients/day were used to evaluate prescription trends. The combinations of these drugs in actual application as well as treatment regiments that are frequently used in clinical practice (monotherapy or combinational therapy) were evaluated. All data in this study were processed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
We enrolled 34,654 adult patients from January 1, 2015 to June 30, 2017 that had a discharge diagnosis of AP. Of these patients, more than 60% were treated in large hospitals (bed number >1,000 beds). Mean age of patients was 49.94 years (95% CI: 49.76–50.11), 62.95% were male patients, median length of hospitalization was 10 days (IQR: 7–14), and hospital mortality rate was 0.35%. After patients were classified according to our definitions for AP severity, number of MAP, MSAP, and SAP patients was 23,100 (66.66%), 10,257 (29.60%), and 1,297 (3.74%), respectively. The stratified data revealed that SAP patients tend to seek medical attention in large hospitals, and mortality, length of hospitalization, and hospitalization costs significantly increased with older age and more severe chronic comorbidities (Table 1).
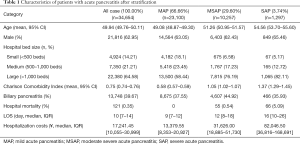
Full table
Study results
- Time of first drug usage: for most AP patients, SSTA and TSI treatment began within 24 hours after admission and were less affected by disease severity.
- Drug treatment: for drug selection, 55% of MAP patients received octreotide as initial treatment, while MSAP and SAP patients mainly selected somatostatin, with utilization rates of 53.66% and 64.61%, respectively. A greater proportion of patients with higher severity received ulinastatin and somatostatin.
- Drug treatment duration: treatment duration for the 4 drugs were similar; however, duration was shorter in MAP patients (around 4 days) and longer in SAP patients (1 week).
- Drug therapeutic index: the therapeutic index of octreotide was lower than that of the other 3 drugs. When disease worsened in patients, the dose used for these drugs increased, particularly ulinastatin; the DUI of ulinastatin in SAP patients was 2.20, which exceeded the recommended dose by 2-fold (Table 2).
- Most frequent medication combinations for different disease severities: for AP patients, physicians tended to employ SSTA monotherapy, such as octreotide or somatostatin (>40%). In addition, the combination of these two drugs with ulinastatin or in the absence of TSI is also an option. After AP worsens, physicians tended to combine SSTAs and TSIs and somatostatin or octreotide. To add, ulinastatin is usually the most common option for SAP patients (>30%) (Table 3).
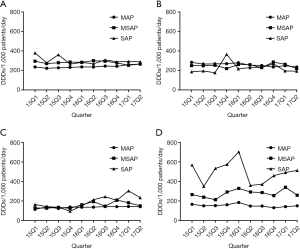
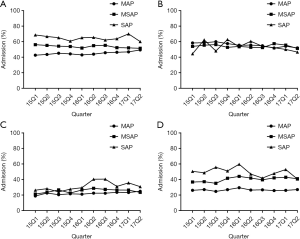
- Analysis of the temporal trends of consumption and drug utilization rates of different drug prescriptions: by examining the prescription volume (expressed as DDDs/1,000 patients/day) for every quarter from January 1, 2015 to June 30 2017, we found that drug consumption for MAP and MSAP was relatively constant compared to that for SAP patients. To add, ulinastatin was found to differ from the other drugs as its prescription consumption was more significant with different disease severities, and its use in SAP patients was significantly higher than that for other drugs. For the temporal trend for utilization rate, with the exception of gabexate in SAP patients, the usage of other drugs was relatively stable during the 2.5-year observation period. Similarly, we observed that the utilization rate of somatostatin and ulinastatin changed with disease severity (Figures 2,3).
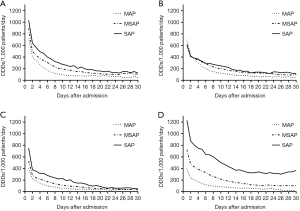
- Drug usage dynamic changes during hospitalization: lastly, we classified the daily drug usage status for every patient during hospitalization. Thereafter, patient data were grouped according to their length of hospitalization (e.g., all patients were hospitalized on Day 1, and study subject was TSI usage status of 34,654 patients on Day 1) and prescription volume of physicians was determined. Among MAP and MSAP patients, the drug with the highest initial prescription volume was somatostatin, with a prescription volume of 723 and 894 DDDs/1,000 patients/day, respectively. In SAP patients, the drug with the highest initial prescription volume was ulinastatin, with a prescription volume of 1,230 DDDs/1,000 patients/day. As treatment duration increased, the consumption of all drugs showed a decreasing trend. However, the consumption of ulinastatin at the later stages of SAP treatment was significantly higher than that of any other drug, which was maintained at 300–400 DDDs/1,000 patients/day (Figure 4).
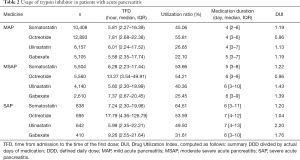
Full table
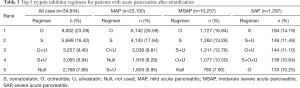
Full table
Discussion
Stratification by AP characteristics and severity
To conduct this study, we used the national database of China to explore the disease characteristics and current status of major drugs for AP in China. Currently, only few large-scale studies on AP exists in China and to our knowledge, this is the first epidemiological study with more than 10,000 AP patients in China. Based on our study results, mean age (SD) of AP patients in China is 49.9 (16.5) years, a value slightly lower than that of most European countries. To add, 63% of patients were males, a value higher than that found for US patients (47%) (12). AP was identified to be caused by cholelithiasis in 40% of patients, which is similar to the ratio reported in other studies (13). In the present study, median hospital mortality rate and length of hospitalization were 0.35% and 10 days, respectively, and in the US, the values were 0.7% and 3 days, respectively (2). Although the length of hospitalization of Chinese patients was longer than that of most countries, the single hospitalization cost of these patients was lower than that for developed countries (median hospitalization cost was 17,241 CNY, which is equivalent to 2,597 US dollars. In 2014, median hospitalization cost was 6,240 US dollars in the US), and hospital mortality rate was lower than the data reported in other countries. Because of the effects of outside hospital mortality, some critically ill or fulminant patients may have died before diagnosis and some may have been prematurely discharged after resuscitation failure, which are common scenarios in China. After patients were classified as MAP, MSAP, or SAP, we found that age and chronic comorbidities were associated with disease severity and the relationship between gender and disease severity was not significant, aligning with the current consensus.
Use of TSI during AP treatment
This real-world study revealed that overall, SSTAs and TSIs are mainstay treatment measures for AP treatment in China; this is despite their unpopular status in the treatment guidelines of the US and Europe (14,15). Chinese physicians prescribe one or more TSIs for more than 90% of AP patients, and treatment with these drugs usually begin immediately after admission. The 48-h period after admission is crucial for AP treatment as patients tend to develop systemic inflammatory response syndrome, organ failure, and other systemic complications during this period. Treatment with SSTAs and TSIs usually lasts 1 week. As the Revised Atlanta Classification for Acute Pancreatitis defines the first week of AP as the early phase (5), the above drugs tend to be used in the early phase of AP. For the early phase of AP, physicians tend to use somatostatin and octreotide and large doses of ulinastatin tend to be given to patients, particularly SAP patients. This is because some published papers showed that SSTAs and ulinastatin are beneficial for patients with AP (16-18). For drug combinations, combining SSTAs and ulinastatin appears as a routine combination used by physicians. However, when disease is mild, SSTA monotherapy is more widely used. This finding might be because SSTAs can inhibit pancreatic secretion, while ulinastatin inhibits interleukin and other inflammatory factors. Together, they exhibit pharmacological synergy and are thus frequently used.
From January 2015 to June 2017, overall prescription volume and patient utilization rate for the patients were stable, indicating that the usage of these drugs was less affected. Significant fluctuations in drug consumption and utilization rate were found when these drugs were used in SAP patients; however, this may be due to the low number of patients used in the study. A significant difference was found in the usage of somatostatin and ulinastatin based on disease severity. To add, this drug combination may be an easy option for SAP patients. As drug consumption was found to trend after patients were admitted, this further validated the use of these drugs at the early stage of the disease.
Interestingly, we found that in addition to a large increase in the consumption of ulinastatin after disease worsening, a high prescription volume of ulinastatin remains at the later stages of disease progression [1 week after admission; studies have shown that persistent organ failure is the key determinant of mortality in AP, which aggravates the occurrence and progression of multiple organ dysfunction syndrome. Persistent resulted in a mortality of 25% compared to 8% with transient systemic inflammatory response syndrome (19,20)]. The most common cause of death 2 weeks after AP onset is usually sepsis and its complications (21,22). Studies have proven that the use of ulinastatin in SAP patients can significantly reduce the incidence and mortality rate of new organ failure (9,23). As a trypsin inhibitor, in addition to effectively inhibiting inappropriately-activated trypsin, ulinastatin can also effectively inhibit the secretion of many inflammatory factors and lysosomes. Ulinastatin can bind to calcium ions to inhibit the influx of calcium ions and block the TNF-a signaling pathway. Ulinastatin can also stabilize the cell membrane and lysosomal membrane to effectively inhibit the release of many hydrolases in lysosomes to block the inflammatory cascade and reduce cell damage (24-27). Studies have also proven that ulinastatin can effectively decrease the 28-day all-cause mortality of severe sepsis and septic shock, reduce the incidence of new multiple organ dysfunction syndrome, and shorten the length of hospitalization (28). These real-world clinical results prove that enzyme inhibition and anti-inflammatory drugs are important therapeutic measures based on the pathophysiological changes of AP (29-31). In SAP treatment, sufficient dose and duration of ulinastatin can benefit patients.
Study strengths and limitations
Real-world studies serve as current drug research hotspots; however, they exist in the preliminary exploratory stage in China. The incidence of AP is less than 1/1,000 and requires a large population to obtain large-scale results. The statistical data obtained from this study can however supplement this research domain. The present study had major limitations. Similar to other retrospective database studies, there were errors and omissions during the retrieval of patient diagnosis or comorbidity data based on the ICD-10th diagnosis code. To add, we could not review patients because of the characteristics of the database itself. When patients were being stratified, complete matching with the classification criteria presented in the guidelines was not achieved as some SAP patients were classified as MSAP patients. Lastly, the dose used to treat AP was selected by default. Octreotide and other drugs may be used for gastric varices, which will result in errors when calculating dosing.
Conclusions
In summary, SSTAs and TSIs are the major therapeutic drugs for AP treatment and are widely utilized in China at the early stage of the disease, with a treatment cycle lasting 1 week. Although somatostatin and octreotide are the most commonly-used drugs, ulinastatin is widely used for SAP and the late stage of AP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE Reporting Checklist. Available at http://dx.doi.org/10.21037/apm-19-363
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-19-363
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-363). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). The clinical research ethics boards of the Changhai Hospital waived the needs for participants’ informed consent because the study was retrospective, anonymous, and non-interventional. All methods in this study were performed in accordance with the relevant regulations and guidelines and the patients’ personal data had been well protected. The review and approval of this study was exempted by the ethics committee of Changhai Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016;1:45-55. [Crossref] [PubMed]
- Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology 2019;156:254-72.e11. [Crossref] [PubMed]
- Wadhwa V, Patwardhan S, Garg SK, et al. Health Care Utilization and Costs Associated With Acute Pancreatitis. Pancreas 2017;46:410-5. [Crossref] [PubMed]
- Munigala S, Subramaniam D, Subramaniam DP, et al. Predictors for early readmission in acute pancreatitis (AP) in the United States (US) - A nationwide population based study. Pancreatology 2017;17:534-42. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Gastroenterology Chinese Medical Association credits will Pancreatology group. Chinese guidelines for the management of acute pancreatitis(Shenyang,2019). Chinese Journal of Pancreatology 2019;19:321-31.
- Chinese Study Group for Pancreatology of Chinese Medical Doctor Associations. Chinese MDT Consensus for the management of acute pancreatitis. Chinese Journal of Pancreatology 2015;15:217-24.
- Horibe M, Egi M, Sasaki M, et al. Continuous Regional Arterial Infusion of Protease Inhibitors for Treatment of Severe Acute Pancreatitis: Systematic Review and Meta-Analysis. Pancreas 2015;44:1017-23. [Crossref] [PubMed]
- Lagoo JY, D'Souza MC, Kartha A, et al. Role of Ulinastatin, a trypsin inhibitor, in severe acute pancreatitis in critical care setting: A retrospective analysis. J Crit Care 2018;45:27-32. [Crossref] [PubMed]
- Nie XM, Li YS, Yang ZW, et al. Initial empiric antibiotic therapy for community-acquired pneumonia in Chinese hospitals. Clin Microbiol Infect 2018;24:658.e1-e6. [Crossref] [PubMed]
- World Health Organization. Defined daily dose 2020. Available online: http://www.who.int/medicines/regulation/medicines-safety/toolkit_ddd/en/
- Sellers ZM, MacIsaac D, Yu H, et al. Nationwide trends in acute and chronic pancreatitis among privately insured children and non-elderly adults in the United States, 2007-2014. Gastroenterology 2018;155:469-78.e1. [Crossref] [PubMed]
- Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med 2016;375:1972-81. [Crossref] [PubMed]
- Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013;108:1400-15; 1416.
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13:e1-e15. [Crossref] [PubMed]
- Wang G, Liu Y, Zhou SF, et al. Effect of Somatostatin, Ulinastatin and Gabexate on the Treatment of Severe Acute Pancreatitis. Am J Med Sci 2016;351:506-12. [Crossref] [PubMed]
- Guo H, Chen J, Suo D. Clinical efficacy and safety of ulinastatin plus octreotide for patients with severe acute pancreatitis. Zhonghua Yi Xue Za Zhi 2015;95:1471-4. [PubMed]
- Wang J, Jin J, Huang J, et al. Clinical value of the early use of ulinastatin in patients with moderately severe or severe acute pancreatitis. Zhonghua Yi Xue Za Zhi 2017;97:1252-5. [PubMed]
- Mofidi R, Duff MD, Wigmore SJ, et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg 2006;93:738-44. [Crossref] [PubMed]
- Mole DJ, Olabi B, Robinson V, et al. Incidence of individual organ dysfunction in fatal acute pancreatitis: analysis of 1024 death records. HPB (Oxford) 2009;11:166-70. [Crossref] [PubMed]
- Gloor B, Muller CA, Worni M, et al. Late mortality in patients with severe acute pancreatitis. Br J Surg 2001;88:975-9. [Crossref] [PubMed]
- Mutinga M, Rosenbluth A, Tenner SM, et al. Does mortality occur early or late in acute pancreatitis? Int J Pancreatol 2000;28:91-5. [Crossref] [PubMed]
- Abraham P, Rodriques J, Moulick N, et al. Efficacy and safety of intravenous ulinastatin versus placebo along with standard supportive care in subjects with mild or severe acute pancreatitis. J Assoc Physicians India 2013;61:535-8. [PubMed]
- Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci 2003;116:1863-73. [Crossref] [PubMed]
- Kanayama N, Maehara K, Suzuki M, et al. The role of chondroitin sulfate chains of urinary trypsin inhibitor in inhibition of LPS-induced increase of cytosolic free Ca2+ in HL60 cells and HUVEC cells. Biochem Biophys Res Commun 1997;238:560-4. [Crossref] [PubMed]
- Kanayama S, Yamada Y, Onogi A, et al. Bikunin suppresses expression of pro-inflammatory cytokines induced by lipopolysaccharide in neutrophils. J Endotoxin Res 2007;13:369-76. [Crossref] [PubMed]
- Atal SS, Atal S. Ulinastatin - a newer potential therapeutic option for multiple organ dysfunction syndrome. J Basic Clin Physiol Pharmacol 2016;27:91-9. [Crossref] [PubMed]
- Karnad DR, Bhadade R, Verma PK, et al. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study. Intensive Care Med 2014;40:830-8. [Crossref] [PubMed]
- Harper SJ, Cheslyn-Curtis S. Acute pancreatitis. Ann Clin Biochem 2011;48:23-37. [Crossref] [PubMed]
- Shrivastava P, Bhatia M. Essential role of monocytes and macrophages in the progression of acute pancreatitis. World J Gastroenterol 2010;16:3995-4002. [Crossref] [PubMed]
- Zhang C, Wang Y, Fu W, et al. A Meta-analysis on the Effect of Ulinastatin on Serum Levels of C-Reactive Protein, Interleukin 6, and Tumor Necrosis Factor Alpha in Asian Patients with Acute Pancreatitis. Genet Test Mol Biomarkers 2016;20:118-24. [Crossref] [PubMed]


