
Application of shear wave elastography as a diagnostic method for esophageal varices
Introduction
Rupture of esophageal varices (EV) is the most common cause of upper gastrointestinal bleeding in cirrhosis, with an incidence reported as high as 50% in the literature. The mortality rate of EV rupture bleeding is 20–30%, with shock and even death most often being caused by untimely treatment (1-3). Consequently, early detection and intervention can reduce the risk of EV rupture and ultimately reduce mortality. The current gold standard for the discovery of EV is endoscopic examination, as recommended by the United States and European Union for all patients with cirrhosis (4,5). However, this examination causes patient discomfort and is expensive, meaning that follow-up requirements often cannot be met.
Another clinical approach for the detection of EV is ultrasound elastography, which can be used to objectively and quantitatively analyze liver stiffness (LS). This procedure is mainly conducted using transient elastography (TE), acoustic radiation force impulse imaging (ARFI), and real-time shear wave elastography (SWE). As the earliest elastography technique to be applied in clinical practice (6), TE is used for the classification of hepatic fibrosis and to predict the occurrence of diseases such as EV and portal hypertension (7-9). However, this method is prone to measurement errors in patients with obesity, severe ascites, and narrow intercostal spaces. Furthermore, TE lacks 2D imaging capabilities and requires specialized equipment. For these reasons, this method is being gradually replaced by ARFI and SWE.
Although widely used in the diagnostic grading of liver fibrosis (7,10), SWE is seldom used to predict the severity of EV and rupture bleeding. Indeed, previous studies have relied on TE and aspartate aminotransferase (AST) to platelet count ratio index (APRI), rather than SWE, for predicting the occurrence of EV (11,12). The purpose of our present study was to investigate the predictive value of SWE for EV grading and rupture bleeding, and to compare the diagnostic performance of SWE with that of other noninvasive methods.
We present the following article in accordance with the Tripod Reporting Checklist (available at http://dx.doi.org/10.21037/apm-20-306).
Methods
Patients
Between October 2017 and October 2018, 120 patients who were clinically diagnosed with cirrhosis in our hospital were enrolled in this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the medical Ethics Committee of the Affiliated Hospital of Qingdao University, and written informed consent was provided by all study participants. Inclusion criteria required successful measurements of LS, spleen stiffness (SS), and other parameters. Exclusion criteria included hepatic and splenic tumor or other neoplasms (n=24), portal vein thrombosis (n=3), types of cirrhosis other than viral hepatitis B (n=19), and a history of portal hypertension operation (splenic embolization, transjugular intrahepatic portosystemic shunt, splenectomy, etc.) (n=17). In addition, three cases were also excluded due to severe obesity and gastrointestinal gas measurement. Therefore, of the 186 participants initially enrolled, 120 (98 men and 22 women) were ultimately included in the study.
SWE
All patients were examined in the morning in a fasted state using the Supersonic Imagine Aixplorer SC6-1 convex array probe (SuperSonic Imagine SA, Aix-en-Provence, France). The patients were placed in a supine position, and the SWE examination was performed using brightness (B)-mode ultrasound. The elasticity image box was set 1 cm deeper than the Glisson capsule and splenic capsule, and the vascular system was avoided during measurement. Measurements of LS were performed on the right lobe of the liver through the intercostal space, with patient’s right upper limb in maximal abduction. The probe was placed on the right side of the 7th to 9th intercostal space. When the target area of the liver was located, the B-mode ultrasound was switched to the SWE mode for measurement of LS. The patient was asked to hold a breath for about 10 s during the measurement. The measurement was repeated five times, and the results were averaged. Measurements of the splenic diameter and SS were performed between the 9th and 11th intercostal space, with the participant’s left upper limb in maximal abduction. Again, 5 measurements were obtained and averaged. Only patients with a coefficient of variability (CV) <0.3 for the LS and SS values obtained were included in further analyses (13).
Laboratory examination
All laboratory data for each patient, including the APRI (APRI = AST/ASTULN ×100, ASTULN =40 U/L), were obtained on the day of ultrasonography by a seasoned physician (≥20 years of clinical experience) who was blinded to the results of other tests.
Upper gastrointestinal endoscopy
The endoscopy was performed by a clinician (with 14 years of clinical experience) within a week of completing the ultrasound and relevant laboratory examinations. The clinician was also blinded to other data. The diagnosis of EV was made according to the Japanese Standard (14), which classifies EV as F1–F3. A red sign indicates a high-risk factor for rupture and bleeding of EV. Low-risk EVs are defined as F1 without red signs or Child-Pugh class C, while high-risk EVs are defined as F2–F3 or F1 with red signs or Child-Pugh class C. And, Figure 1 is the flow of participants through the study.
Statistical analysis
The statistical software systems SPSS 21.0 (SPSS Inc., IBM, Chicago, IL, USA) and MedCalc15 (MedCalc Software Ltd., Ostend, Belgium) were used for statistical analysis. Variables are represented by median (
Results
Patient characteristics
The endoscopic examinations revealed that 36 patients were non-EV and 84 were EV, of whom 49 were low-risk EV and 35 were high-risk EV. Among the 84 patients with EV, 17 were hemorrhagic and the other 67 were not. The characteristics of all 120 patients are summarized in Table 1.
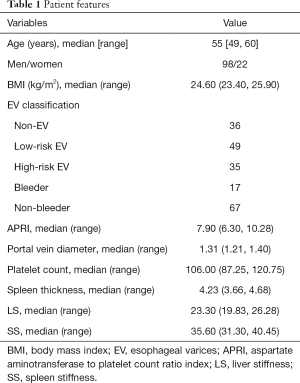
Full table
Diagnostic ability of variables to differentiate the severity of EV
The variables of gender, age, and BMI had P values of 0.548, 0.600, and 0.078, respectively; they could not be used as diagnostic indicators of the presence or absence of EV. By contrast, APRI, portal vein diameter, platelet count, spleen thickness, LS, and SS all had P values <0.05, so they potentially could serve as indicators (Table 2). However, no difference was observed for platelet count and APRI (P=0.301, 0.564, respectively) between patients with low-risk EV versus high-risk EV (Table 3). Only SS was a statistically significant indicator (P<0.001) for the identification of EV with or without bleeding (Table 4).

Full table

Full table
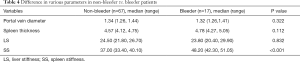
Full table
Comparisons of diagnostic performance among variables
The ROC curve analysis showed AUCs for APRI, portal vein diameter, platelet count, spleen thickness, LS, and SS diagnosed with or without EV of 0.76, 0.81, 0.82, 0.89, 0.84, and 0.91, respectively. In diagnosing EV, SS was superior to APRI, portal vein diameter, platelet count, spleen thickness, and LS (Figure 2A). The AUCs for portal vein diameter, spleen thickness, LS, and SS were 0.67, 0.65, 0.66, and 0.77, respectively, for diagnosing low-risk EV and high-risk EV (Figure 2B). The cutoff values, specificity, sensitivity, Youden index, +LR and −LR of the APRI, portal vein diameter, platelet count, spleen thickness, LS, and SS for diagnosing cirrhosis with or without EV and diagnosing of low-risk EV and high-risk EV are shown in Tables 5,6. In distinguishing EV with hemorrhage or without hemorrhage, the cutoff value of 39.3 KPa for SS had a specificity of 98.60%, sensitivity of 70.15%, Youden index of 0.72, +LR of 3.35, and −LR of 0.01. The distribution of LS and SS in EV classification is shown in Figure 3.
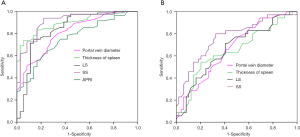
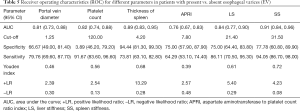
Full table

Full table
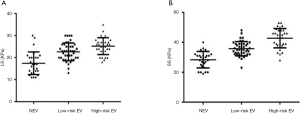
Correlation between variables and the severity of EV
Spearman’s correlation analysis indicated a moderate correlation between the occurrence of EV and APRI, portal vein diameter, platelet count, and LS (r=0.41, 0.49, 0.51, 0.54, P<0.05), and a high correlation with spleen thickness and SS (r=0.63, 0.65, P<0.05).
Discussion
The rupture and bleeding of EV is one of the most serious complications of portal hypertension in cirrhosis, and is the main cause of death under this condition. Especially, the risk of gastrointestinal hemorrhage is higher in patients with moderate and severe EV. Accordingly, great significance has been placed upon the early determination of the existence and severity of EV, prediction of the risk of rupture and bleeding, and timely action of corresponding interventions to reduce mortality and improve the prognosis of patients with cirrhosis. Therefore, an efficient, accurate, and reliable non-invasive detection method has been a crucial need in the evaluation of EV. The emergence of ultrasonic elastic imaging can perhaps fulfill this need, and SWE imaging in particular can quantitatively detect organ hardness and reflect the degree of organ fibrosis.
In this study, APRI, portal vein diameter, platelet count, spleen thickness, LS, and SS were found to be applicable for the diagnosis of EV (P values all <0.05), as their AUC values were 0.76, 0.81, 0.82, 0.89, 0.84, and 0.91, respectively. The LS and SS cutoff values were 21.4 and 31.5 KPa, which is consistent with the values reported by Grgurević et al. (15). Platelet count and APRI were unsuitable for the diagnosis of low-risk EV and high-risk EV (all P values >0.05), in agreement with the findings of Sharma et al. (16) and Shi et al. (17).
The platelet count and APRI were not discriminatory for the diagnosis of low-risk EV versus high-risk EV, and the specificity of platelet count (63.89%) and sensitivity of APRI (64.29%) were not sufficiently high for the diagnosis of EV. Nevertheless, these are all simple and rapid methods for evaluating cirrhosis and for predicting EV, so their clinical value should not be ignored. Of all the parameters assessed, only portal vein diameter, spleen thickness, LS, and SS were able to identify both low-risk EV and high-risk EV. The LS and SS cutoff values were 23.8 and 38.1 KPa, respectively, which were contrary to the values reported by Sharma et al. (16), who dismissed LS as useless in identifying low-risk and high-risk EV. This discrepancy could be a result of our study solely recruiting participants with hepatitis B cirrhosis, and not patients diagnosed with hepatitis B cirrhosis, alcoholic cirrhosis, or other types of hepatic cirrhosis who may present variable LS measurements. In addition, SWE is superior to TE in terms of its diagnostic performance (18). Other influencing study design factors might be differences in sample size and subject nationality. In fact, as by reported by Sporea et al. (19,20), the LS differed between Asian and European patients even for the same type of liver disease and fibrosis classification.
Although the diagnostic value of LS and SS for discriminating between low-risk EV and high-risk EV is contentious and needs further investigation, the preferable diagnostic efficiency of SS versus LS has been confirmed. In addition, the range of increase of SS is higher than that of LS, which may be associated with portal perfusion and spleen perfusion; LS decreases with a decrease in portal perfusion, whereas SS increases with an increase in spleen perfusion (21).
Portal hypertension is a major risk factor for EV bleeding. As suggested by Egawa et al. (22), a hepatic venous pressure gradient (HVPG) >10 mmHg could result in bleeding of EV. However, this is difficult to control in clinical practice because of the invasiveness of the examination. The first evaluation of SWE as a tool for non-invasive diagnosis of EV was performed by Grgurević et al. (15), but these researchers did not investigate the ability of SWE to provide a differential diagnosis of low-risk EV and high-risk EV, or its effectiveness at predicting EV bleeding. In the present study, SS predicted EV bleeding with high diagnostic efficiency (AUC =0.93). For diagnosing rupture bleeding, a high sensitivity and specificity can be achieved with SS at a cutoff value of 39.3 KPa.
In the present study, SWE was confirmed as effective for the diagnosis of EV classification and rupture bleeding. As indicated, both LS and SS increased with the increase in EV, and the increase was higher for SS than for LS, indicating that SS had a better diagnostic performance. The reason for this is that the measurement of LS is affected by liver inflammation, congestion, cholestasis, etc., as well as alanine aminotransferase (ALT) and bilirubin (23) levels. In addition, portal hypertension and hemodynamic changes cannot be accurately measured in practice, and portal hypertension is one of the main risk factors for EV rupture and hemorrhage.
This study had some limitations. Firstly, the sample size was small, and secondly, it was a single-center study. The findings should be verified in future using larger sample sizes and multicenter data.
In summary, SWE has a high predictive value for EV classification and bleeding. The use of SWE for diagnosis can reduce unnecessary endoscopy and portal hypertension monitoring, and thus this method may have considerable value in the diagnosis and treatment of EV.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Tripod Reporting Checklist. Available at http://dx.doi.org/10.21037/apm-20-306
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-306
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-306). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the medical Ethics Committee of the Affiliated Hospital of Qingdao University (No. 2017-89771353), and written informed consent was provided by all study participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology 2001;120:726-748. [Crossref] [PubMed]
- Jensen DM. Endoscopic screening for varices in cirrhosis: findings, implications, and outcomes. Gastroenterology 2002;122:1620-30. [Crossref] [PubMed]
- Hogan BJ, O'Beirne JP. Role of self-expanding metal stents in the management of variceal haemorrhage: Hype or hope? World J Gastrointest Endosc 2016;8:23-9. [Crossref] [PubMed]
- Thomson M, Tringali A, Dumonceau JM. Paediatric Gastrointestinal Endoscopy: European Society for Paediatric Gastroenterology Hepatology and Nutrition and European Society of Gastrointestinal Endoscopy Guidelines. J Pediatr Gastroenterol Nutr 2017;64:133-53. [Crossref] [PubMed]
- de Franchis R, Faculty BV. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762-8. [Crossref] [PubMed]
- Shi KQ, Fan YC, Pan ZZ, et al. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int 2013;33:62-71. [Crossref] [PubMed]
- Berzigotti A, Seijo S, Arena U, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 2013;144:102-111.e1. [Crossref] [PubMed]
- Augustin S, Millán L, González A, et al. Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: a prospective study. J Hepatol 2014;60:561-9. [Crossref] [PubMed]
- Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 2011;140:1970-9. [Crossref] [PubMed]
- Zeng J, Huang Z, Jin J, et al. Diagnostic Accuracy of 2-D Shear Wave Elastography for the Non-Invasive Staging of Liver Fibrosis in Patients with Elevated Alanine Aminotransferase Levels. Ultrasound Med Biol 2018;44:85-93. [Crossref] [PubMed]
- Yang XP. Application Value of Liver and Spleen Stiffness Detected by Transient Elastography for Predicting Esophageal Varices. Chinese Journal of Ultrasound in Medicine 2017;33:139-42.
- Chen M, Zhang DP, Gao YJ, et al. Clinical Value of acoustic radiation force impulse in quantitative prediction of the degree of esophageal varices in patients with liver cirrhosis. J Clin Hepatol 2018;34:80-3.
- Reed GF, Lynn F, Meade BD. Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol 2002;9:1235-9. [PubMed]
- Tajiri T, Yoshida H, Obara K, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc 2010;22:1-9.
- Grgurević I, Bokun T, Mustapić S, et al. Real-time two-dimensional shear wave ultrasound elastography of the liver is a reliable predictor of clinical outcomes and the presence of esophageal varices in patients with compensated liver cirrhosis. Croat Med J 2015;56:470-81. [Crossref] [PubMed]
- Sharma P, Kirnake V, Tyagi P, et al. Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am J Gastroenterol 2013;108:1101-7. [Crossref] [PubMed]
- Shi Y, Liu Y, Li QJ, et al. Spin-echo planar imaging MR elastography in evaluation of gastroesophageal varices in liver cirrhosis. Chin J Med Imaging Technol 2016;32:266-9.
- Lee JE, Shin KS, Cho JS, et al. Non-invasive Assessment of Liver Fibrosis with ElastPQ: Comparison with Transient Elastography and Serologic Fibrosis Marker Tests, and Correlation with Liver Pathology Results. Ultrasound Med Biol 2017;43:2515-21. [Crossref] [PubMed]
- Sporea I, Bota S, Peck-Radosavljevic M, et al. Acoustic Radiation Force Impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: an international multicenter study. Eur J Radiol 2012;81:4112-8. [Crossref] [PubMed]
- Sporea I, Bota S, Grădinaru-Taşcău O, et al. Comparative study between two point Shear Wave Elastographic techniques: Acoustic Radiation Force Impulse (ARFI) elastography and ElastPQ. Med Ultrason 2014;16:309-14. [PubMed]
- Warner L, Yin M, Glaser KJ, et al. Noninvasive In vivo assessment of renal tissue elasticity during graded renal ischemia using MR elastography. Invest Radiol 2011;46:509-14. [Crossref] [PubMed]
- Egawa H, Nishimura K, Teramukai S, et al. Risk factors for alcohol relapse after liver transplantation for alcoholic cirrhosis in Japan. Liver Transpl 2014;20:298-310. [Crossref] [PubMed]
- Li Y, Hao ZH, Chen CY, et al. Application Value of Transient Elastography in Nonalcoholic Fatty Liver Disease. Chinese J Ultrasound Med 2014;30:414-8.
(English Language Editors: J. Jones and J. Gray)



