
A prediction model of enteral nutrition complicated with severe diarrhea in ICU patients based on CD55
Introduction
Severe diarrhea is the leading cause of electrolyte disturbance worldwide and the main cause of suspension of enteral nutrition in hospitals (1), but the best diagnosis strategy remains uncertain. In particular, there is evidence that discontinuation of enteral nutrition and general antidiarrheal therapy is not ideal, and may cause nutritional deficiencies, delay recovery, and even increase hospital stay or mortality in some patients (2). Therefore, early detection of enteral nutrition-induced severe diarrhea and timely treatment can improve the clinical prognosis of patients with severe diarrhea.
Uncontrolled complement activation may be of immunopathological importance in inflammatory diseases of the gastrointestinal tract (3). CD55 is one of these regulatory membrane proteins of the complement system. CD55 not only plays a vital role in immune but also plays a crucial role in intestinal function (4-7). Studies in animal models have shown that CD55 has a protective effect on the intestinal function, primarily through anti-inflammatory activity. Endogenous CD55 dysfunction worsens intestinal function injury in animal models (8). In clinical studies, low CD55 levels increased the incidence of protein-losing enteropathy in some patients (9-11). However, further studies are needed to confirm the protective effects of CD55 in enteral nutrition-induced severe diarrhea. Although defecate frequency can be used to diagnose severe diarrhea development, these assessments are often too late to discover severe diarrhea. To solve this decision-making problem, we wanted to design a diagnostic model to extract relevant intestinal barrier indicators from cases. We intended to build a prediction model of enteral nutrition complicated with severe diarrhea in ICU patients based on CD55.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1050).
Methods
Design and setting
This monocentric, prospective observational study was conducted in one teaching hospital (Shanghai General Hospital, Shanghai, China) from January 1th, 2019 to January 31th, 2020 with recruitments periods form January 31th, 2019 to December 2th, 2019. Patients’ follow up ended on January 31th 2020 with data collection periods from January 31th 2019 to January 31th 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of the Shanghai General Hospital {reference number [2018]KY004}. Since the data collected was anonymous, the need for informed consent was waived.
Patients
Patients admitted to the ICU of Shanghai General Hospital (Shanghai, China) with enteral nutrition were included in the study. Inclusion criteria for the patients were as follows: patients between 60 to 85 years old who underwent enteral nutrition in the ICU, with an estimated length of stay in the ICU of >48 h. Exclusion criteria for the patients were as follows: history of gastrointestinal surgery, chronic diarrhea, pregnancy, lactation period hemodynamic instability, tumor, at the same time to participate in another interventional clinical trials.
Data collection
Baseline data, including demographics, medical history, and disease severity, were collected prospectively for each patient when enrolled. Diagnostic criteria: severe diarrhea was defined as defecation frequency ≥3 per day or change of fecal characteristics. The change of fecal characteristics was documented in nursing notes and medical history. We used a classification system (Bristol Stool Chart). Nurses and doctors documented this information. They were trained before this trail, including data management, defined procedures.
Vital signs, defecation frequency, change of fecal characteristics were collected each day during the ICU stay. Laboratory data were collected on days 1 and 7 after enrollment, such as intestinal barrier function improvement (diamine oxidase, D lactic acid and endotoxin), CD55 and IL-10. The routine medical records system of the hospital was used to register the data for follow-up, if discharged, telephone follow-up.
CD55 detection method
CD55 expression of neutrophils in peripheral blood was detected by flow cytometry. The cells were washed with phosphate buffer (PBS), centrifuged, the supernatant was discarded, and the cell suspensions were resuspended with 100 µL PBS. The cell suspensions were added to flow tubes. Monoclonal antibody from mice (Mouse IgG2a, κ; BD Pharmingen™), who specifically binds to CD55, were added for 3 microns and incubated at 4 °C in dark for 15 min; add 1 mL of RBC (red blood cell) lysate, hide from light for 10 min, wash with PBS, re-suspend, and test on machine. All antibodies and erythrocyte lysates are from BD company, flow cytometer (FACSCalibur) and its supporting software are products of BD company. It was the same person who performed the flow cytometry.
Outcome
The primary outcome was severe diarrhea, the secondary outcomes included 28-day mortality, length of hospital stay (days).
Statistical analysis
Study size
We didn’t calculate the sample size because this was an observational descriptive study. Data analyses were performed using Statistical Package for Social Sciences software for Windows, version 16.0 (SPSS Inc, Chicago, IL, USA), and P values <0.05 were considered statistically significant. Quantitative values that conform to normal distribution are expressed by mean ± standard deviation, while those that do not are expressed by median (quartile). The enumeration data are described by various cases and percentages. The difference of CD55 level between diarrhea group and control group was compared by Mann-Whitney U test.
Univariate and multivariate conditional logistic regression models were used to screen independent influencing factors, and the OR value and 95% confidence interval of the risk of diarrhea were calculated.
Through multi-factor logistic regression model, a prediction model based on multiple prediction indicators was formed, and new joint predictive factors were calculated. With diarrhea or not as the outcome, the area under the receiver operating characteristic curve (AUROC) of the combined predictors and each original index was compared to determine the optimal critical value, calculate the operating performance parameters such as sensitivity, specificity and accuracy of prediction, and finally carry out individual prediction by substituting individual values. Stata 10.0 software was used for statistical analysis and mapping, and Stata 10.0 command statement, operation process and result output. There was no loss of follow-up and no missing date.
Results
General information
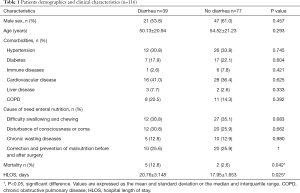
One hundred and twenty-four patients were eligible of the study, but 8 had to be excluded (Figure 1). In this series, there were 77 without severe diarrhea patients and 39 severe diarrhea patients. Their median age was 53.04 years (range, 18 to 65 years); 68 patients were male and 107 had a history of chronic disease. The diarrhea and no-diarrhea group did not differ in age, gender, incidence of chronic disease and cause of need enteral nutrition (Table 1). The incidence of mortality was higher in the diarrhea group than in the no diarrhea group (12.8% vs. 2.6%, P=0.042). Hospital length of stay (HLOS) was longer in the diarrhea group than in the no diarrhea group (20.76±3.148 vs. 17.95±1.653, P=0.025) (Table 1).
Full table
Analysis of individual factors
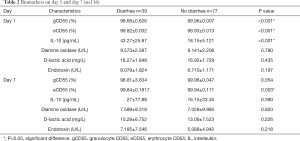
There was significant difference in gCD55 (98.68%±0.626% vs. 99.96%±0.007%, P<0.001), eCD55 (99.82%±0.032% vs. 99.93%±0.013%, P<0.001), and IL-10 (43.27±25.87 vs. 18.15±5.121 pg/mL, P<0.001) between the two groups in day 1. But only eCD55 was significant difference in day 7 (99.84%±0.1817% vs. 99.94%±0.111%, P=0.003). There was no significant statistical difference between the two groups of diamine oxidase in day 1 (9.573±2.587 vs. 9.141±2.206 U/L, P=0.780) and day 7 (7.589±9.318 vs. 7.028±9.985 U/L, P=0.820).
There was no significant difference in D-lactic acid between the two groups in day 1 (18.27±1.848 vs. 16.92±1.729 mg/L, P=0.435) and day 7 (15.29±6.752 vs. 13.06±7.523 mg/L, P=0.226).
There was no significant difference in endotoxin between the two groups in day 1 (8.079±1.824 vs. 6.710±1.171 U/L, P=0.197) and day 7 (7.185±7.546 vs. 5.088±4.942 U/L, P=0.218) (Table 2).
Full table
Independent risk factors for diarrhea condition
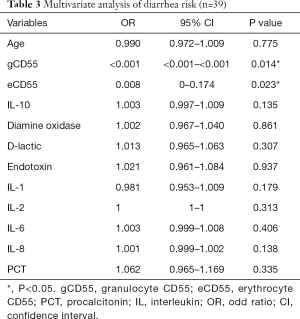
Multivariate logistic regression revealed two independent risk factors for severe diarrhea: gCD55 (P<0.05), eCD55 (P<0.05). IL-10, diamine oxidase, D-lactic, Endotoxin, IL-1, IL-2, IL-6, IL-8 and PCT were not risk factors for severe diarrhea (Table 3).
Full table
The granular membrane surface CD55 of patients with enteral nutrition complicated with refractory diarrhea was significantly lower than that of patients without diarrhea (98.68%±3.91% vs. 99.96%±0.06%, P<0.0001) (Figure 2A).
CD55 on erythrocyte membrane surface in patients with diarrhea was significantly lower than that in patients without diarrhea (99.82%±0.20% vs. 99.93%±0.12%, P<0.0001) (Figure 2B).
Serum interleukin-10 (IL-10) in patients with diarrhea was significantly higher than that in patients without diarrhea (43.27±25.88 vs. 18.15±5.121 pg/mL, P<0.0001) (Figure 2C). Both groups show no significant difference in diamine oxidase, D lactic acid and endotoxin level (Figure 2D,E,F).
Diagnostic value of CD55
When AUROC =0.919 and cutoff value =99.95%, the optimal sensitivity and specificity were 96.2% and 100%, respectively. The AUROC of erythrocyte membrane surface CD55 in predicting diarrhea was 0.658, and the AUROC of IL-10 in predicting diarrhea was 0.558 (Figure 3).

Comparetion of HLOS in two groups
HLOS in patients with diarrhea was significantly higher than that in patients without diarrhea (20.76±3.148 vs. 17.95±1.653 days, P=0.025) (Figure 4).
Formulation and evaluation of the prediction model
Based on this, a prediction model was established. Formulation and evaluation of the prediction model: Witch values (day 1) were used for the prediction model. The prediction model was: In[P/(1− P)] =−24.68 gCD55 − 2.26 eCD55 + 0.0041 IL-10 +2,691, the goodness of fit test of the model was P=0.44, and the area under the curve of the ROC curve of the model was 0.92 (Figure 5). The model showed a good degree of calibration and differentiation.
Discussion
Diarrhea is a common complication of enteral nutrition in severe patients, 50–63% of which occur during nasogastric tube diet (1). Our study found that diarrhea accounted for 53.6% of enteral nutrition during nasogastric tube, which was consistent with the findings. Clinical problems such as electrolyte disorder, fecal incontinence and pressure ulcers brought by diarrhea to ICU patients all increase their medical expenses. The main treatment methods are to stop enteral nutrition, stop diarrhea, regulate intestinal flora, etc. When the nutritional support is interrupted, it will further increase the risk of nutritional deficiency/malnutrition in patients, and ultimately affect the recovery and prognosis of patients. Our study found that the hospitalization time of the diarrhea group was significantly longer than that of the non-diarrhea group. Mortality was also significantly higher in the diarrhea group than in the non-diarrhea group. This is consistent with the recent international nutrition guidelines (12), early improvement of enteral nutrition combined with diarrhea treatment can benefit survival, and a number of RCT studies have reported that patients with early enteral nutrition have higher survival rate, and a number of studies have reported that nutritional standards are related to the prognosis of severe patients (2,13). Based on the results of these studies, we speculate that early enteral nutrition and nutritional standards are beneficial to patients with severe diseases, while enteral nutrition causes diarrhea, which not only affects the timing of enteral nutrition, but also affects the standards of enteral nutrition, so early detection and timely treatment of refractory diarrhea is crucial.
Currently, most traditional indicators, such as diamine oxidase, endotoxin and D-lactic acid, are used to determine the severity of intestinal barrier damage and thus serve as an important basis for starting enteral nutrition. However, these indicators can only reflect the changes in the intestinal barrier function and often lack specificity (14-16). Our study found that there was no significant difference between the diarrhea group and the non-diarrhea group in the differences of the diamine oxidase, endotoxin and D-lactic acid. Therefore, we need to diagnose severe diarrhea earlier through other auxiliary indicators, such as damage molecular markers, because it is more likely to require other corresponding treatment. In contrast, enteral nutrition can be timely applied to early improved diarrhea, so biomarkers that can predict the recovery of intestinal function can be used to guide clinical decisions.
CD55 is an early marker that can be used to reflect intestinal homeostasis (17,18) and can be expressed in heterogeneous adult ICU population and help early identification of patients at high risk of diarrhea. The New England journal has reported that the loss of CD55 protein function can cause life-threatening intestinal inflammation, chronic diarrhea and vascular embolism (4,5). The team, led by Kaan Boztug and Michael Lenardo, proposed that the deletion of the CD55 protein inhibits the proper control of the complement system, which is an important part of innate immune defense and induces pro-inflammatory cytokine signaling. At the same time, researchers have shown that the loss of CD55 results in impaired production of IL-10, a key anti-inflammatory signaling molecule in the intestine (5). The combination of these effects leads to a potentially life-threatening clinical disease in which the overfunctioning complement system collapses in the absence of regulatory IL-10 molecules. The observed intestinal inflammation is a direct consequence of the continued stimulation of immune cells throughout the intestinal flora, and fine-regulated regulatory mechanisms are particularly important here. Inflamed lymphatic vessels in the intestine result in loss of proteins through the plasma, a major symptom of CD55 deficiency, along with the risk of thrombosis. To date, the role of CD55 in maintaining intestinal homeostasis has been largely unexplored (19). Similarly, the underlying molecular mechanisms by which intestinal protein loss plays a role in a large number of diseases are widely unknown.
Our study found that CD55 on granule membrane surface of diarrhea patients was significantly lower than that of patients without diarrhea, and CD55 on erythrocyte membrane surface of diarrhea patients was significantly lower than that of patients without diarrhea, plasma IL-10 in patients with diarrhea was significantly higher than that in patients without diarrhea. AUROC =0.919 when CD55 on the surface of granule membrane predicted the area under the curve of diarrhea, when cutoff value =99.95%, the optimal sensitivity was 96.2%, and specificity was 100%. The AUROC value of erythrocyte membrane surface CD55 in predicting diarrhea was 0.658, and the AUROC value of IL-10 in predicting diarrhea was 0.558 (Figure 2). Based on this, a prediction model was established. The prediction model was: In[P/(1− P)] =−24.68 gCD55 − 2.26 eCD55 + 0.0041 IL-10 +2,691, the goodness of fit test of the model was P=0.44, and the area under the curve of the ROC curve of the model was 0.92. Compared with the traditional intestinal barrier biomarkers such as diamine oxidase, D-lactic acid and bacterial endotoxin, CD55 on the membrane surface of granule showed better prediction effect. The prediction model based on CD55 and combined with IL-10 also has good predictive value. Therefore, we concluded that the decrease of CD55 on granule membrane surface has certain predictive value for enteral nutrition complicated with diarrhea. This provides preliminary data/evidence for other clinical studies on intestinal biomarkers in the future.
Explain its level and influence, and briefly explain its scientific significance or application prospects:
Limitations
This study was a single-center, observational study. The sample size was not enough to verify the model. Diarrhea could be induced by several factors, i.e., the daily caloric intake, the flow rate of the tube feeding, the feeding composition, medication (in particular antibiotics), morbidity of the patient, infection with clostridium difficile. However, the data in this study were statistically significant in predicting severe diarrhea. Because of the study period, we are unable to analyze the long-term prognosis of the enrolled patients, but our study analyzed the short-term prognosis. We thought the generalisability (external validity) of the study results was worth expecting multi-center studies and data from a larger sample are needed to validate our conclusions.
Conclusions
The decrease of gCD55 and eCD55 had certain predictive value in enteral nutrition complicated with diarrhea. The model established by combining gCD55, eCD55 and IL-10 showed a good degree of calibration and differentiation. It provided reference value for early detection of severe diarrhea.
Acknowledgments
We thank all the staff for their valuable contribution to the study.
Funding: This project was supported by grants from Wu Jieping Medical Foundation (No. 320.6750.18546), Songjiang District Science and Technology Project (No. 19SJKJGG92), Shanghai Science and Technology Committee Scientific and Technological Support Project (No. 18411950600 and No. 18411950602), Clinical Research Innovation Plan of Shanghai General Hospital (No. CTCCR-2016B01).
Footnote
Reporting Checklist: The authors have completed the STROBE guideline checklist. Available at http://dx.doi.org/10.21037/apm-20-1050
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1050
Peer Review File: Available at http://dx.doi.org/10.21037/apm-20-1050
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1050). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was obtained from Shanghai General Hospital Institutional Review Board {reference number [2018]KY004}. Since the data collected was anonymous, the need for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Homann HH, Kemen M, Fuessenich C, et al. Reduction in diarrhea incidence by soluble fiber in patients receiving total or supplemental enteral nutrition. JPEN J Parenter Enteral Nutr 1994;18:486-90. [Crossref] [PubMed]
- Elke G, Wang M, Weiler N, et al. Close to recommended caloric and protein intake by enteral nutrition is associated with better clinical outcome of critically ill septic patients: secondary analysis of a large international nutrition database. Crit Care 2014;18:R29. [Crossref] [PubMed]
- Berstad AE, Brandtzaeg P. Expression of cell membrane complement regulatory glycoproteins along the normal and diseased human gastrointestinal tract. Gut 1998;42:522-9. [Crossref] [PubMed]
- Ozen A, Comrie WA, Ardy RC, et al. CD55 Deficiency, Early-Onset Protein-Losing Enteropathy, and Thrombosis. N Engl J Med 2017;377:52-61. [Crossref] [PubMed]
- Kurolap A, Eshach-Adiv O, Baris HN. CD55 Deficiency and Protein-Losing Enteropathy. N Engl J Med 2017;377:1500. [Crossref] [PubMed]
- Kurolap A, Eshach-Adiv O, Hershkovitz T, et al. Loss of CD55 in Eculizumab-Responsive Protein-Losing Enteropathy. N Engl J Med 2017;377:87-9. [Crossref] [PubMed]
- Pan J, Zhang L, Odenwald MA, et al. Expression of human decay-accelerating factor on intestinal epithelium of transgenic mice does not facilitate infection by the enteral route. J Virol 2015;89:4311-8. [Crossref] [PubMed]
- Koretz K, Brüderlein S, Henne C, et al. Decay-accelerating factor (DAF, CD55) in normal colorectal mucosa, adenomas and carcinomas. Br J Cancer 1992;66:810-4. [Crossref] [PubMed]
- Andoh A, Fujiyama Y, Sumiyoshi K, et al. Interleukin 4 acts as an inducer of decay-accelerating factor gene expression in human intestinal epithelial cells. Gastroenterology 1996;111:911-8. [Crossref] [PubMed]
- Lu X, Li Y, Simovic MO, et al. Decay-accelerating factor attenuates C-reactive protein-potentiated tissue injury after mesenteric ischemia/reperfusion. J Surg Res 2011;167:e103-e115. [Crossref] [PubMed]
- Weeks C, Moratz C, Zacharia A, et al. Decay-accelerating factor attenuates remote ischemia-reperfusion-initiated organ damage. Clin Immunol 2007;124:311-27. [Crossref] [PubMed]
- McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159-211. [Crossref] [PubMed]
- Compher C, Chittams J, Sammarco T, et al. Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit Care Med 2017;45:156-63. [Crossref] [PubMed]
- Fukuda T, Tsukano K, Nakatsuji H, et al. Plasma diamine oxidase activity decline with diarrhea severity in calves indicating systemic dysfunction related to intestinal mucosal damage. Res Vet Sci 2019;126:127-30. [Crossref] [PubMed]
- Zeng J, Li YQ, Zuo XL, et al. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2008;28:994-1002. [Crossref] [PubMed]
- Ahmed T, Azam MA, Armed N, et al. Detection of endotoxin in sera from children hospitalized for treatment of diarrhea in Bangladesh. J Endotoxin Res 2004;10:223-8. [Crossref] [PubMed]
- Inaba T, Mizuno M, Ohya S, et al. Decay-accelerating factor (DAF) in stool specimens as a marker of disease activity in patients with ulcerative colitis (UC). Clin Exp Immunol 1998;112:237-41. [Crossref] [PubMed]
- Uesu T, Mizuno M, Inoue H, et al. Enhanced expression of decay accelerating factor and CD59/homologous restriction factor 20 on the colonic epithelium of ulcerative colitis. Lab Invest 1995;72:587-91. [PubMed]
- Dalle Lucca JJ, Simovic M, Li Y, et al. Decay-accelerating factor mitigates controlled hemorrhage-instigated intestinal and lung tissue damage and hyperkalemia in swine. J Trauma 2011;71:S151-S160. [Crossref] [PubMed]






