
Neurocognitive improvement after angioplasty in patients with chronic middle cerebral artery stenosis and cerebral ischemia
Introduction
Intracranial artery stenosis or occlusion is an essential factor in causing cerebral ischemic disease (1-3). Recent studies have suggested that stroke in the middle cerebral artery (MCA) each year accounts for 4–15% of stenosis in all patients (4-7). At present, the main treatments for such patients are drugs and surgery. Recent studies have suggested that compared with drug therapy, patients undergoing endovascular stenting can have improved functional outcomes and have a reduction of mortality (5). However, it may cause many complications, such as post-stroke depression, which severely affects clinical prognosis and increase morbidity and mortality (8).
The use of MCA angioplasty, compared with drug therapy, has been controversial. Studies suggest that perioperative complications for intracranial angioplasty are more frequent than those of drug therapy, and the risk of recurrence is also high, even in patients who received angioplasty and after 1-year in their follow-up period (9). Nevertheless, some studies have suggested that patients who receive cerebral artery angioplasty can have improved functional outcomes and a reduction in mortality (5).
Nearly no literature exists concerning the neurocognitive function of patients with middle cerebral artery stenosis (MCAS) and objective cerebral ischemia. Our previous study therefore investigated the protective effect of edaravone on symptomatic intracranial artery stenosis after stent implantation and its relationship with sex hormones (10). The aim of the present study was to evaluate the cognitive function changes in chronic MCAS patients accepted before and after MCA angioplasty and to explore the influence of MCAS and objective cerebral ischemia. We present the following article in accordance with the STROBE Reporting Checklist (available at http://dx.doi.org/10.21037/apm-20-15).
Methods
Patients
All patients were aged 45–65 years. MCAS was documented by transcranial doppler sonography (TCD), computed tomography angiography (CTA), or magnetic resonance angiography (MRA). Objective ipsilateral hemisphere ischemia was documented by perfusion CT with ioversol stress or perfusion MRA with gadodiamide stress. All patients were followed up clinically for at least 3 weeks before angioplasty.
Exclusion criteria
We excluded patients with ischemic stroke within 2 weeks, vascular disease precluding catheter-based techniques, intracranial aneurysm or arteriovenous malformation, any history of a bleeding disorder, any surgery planned within 30 days, life expectancy ≤1 year, education level below an elementary level, aphasia, bilateral limb hemiparesis, marked depression, or moderate or worse dementia.
Our study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee of Renmin Hospital, Wuhan University (No. 2019-X-16). We obtained written, informed consent from each patient or substitute decision-maker.
Neurocognitive function evaluation
Neuropsychological tests were applied within 7 days before and 6-month after angioplasty. Cognitive function evaluation was performed by 2 independent clinical psychologists, who were blinded to the outcome of the intervention. The cognitive assessment used global measures, including the Mini-Mental State Examination (MMSE) (11), Montreal cognitive assessment scale (MoCA) (12), and Multi-Dimensional Psychology. MMSE and MoCA are widely used rating instruments that assesses memory, orientation, language, and constructional praxis. The scores range from 0 to 30, with a higher score indicating better performance. Multi-Dimensional Psychology is a web-based applications available at www.dweipsy.com (13). The test content includes the choice reaction time, Raven’s progressive matrices test, 3-dimensional (3D) mental rotation, simple calculation, spatial working memory, visual tracking task, word memory, and picture memory (14-17). The Multi-Dimensional Psychology test index is presented in percentiles, wth higher scores representing better performances.
Interventional procedures and clinical follow-up
In this angioplasty procedure, all patients received general anesthesia. Next, diagnostic cerebral angiography (DSA) was performed via the femoral route and angioplasty, including balloon dilatation (gateway), self-expanding stent (Enterprise; Wingspan; 9:5). Surgical success was defined as an improvement of the blood perfusion of MCA of at least 1 level, final residual diameter stenosis <50%, and thrombolysis in myocardial infarction grade 3 antegrade flow. All patients were sent to the neurointensive care unit (NICU) for overnight hemodynamic and neurologic monitoring, where systolic blood pressure was carefully maintained between 100 and 130 mmHg. Aspirin and clopidogrel were continued for 3 months after successful intervention, while long-term clopidogrel and lipid-lowering drugs were added according to the patient condition. Complete neurologic examinations, including assessment with the National Institutes of Health Stroke (NIHSS), and modulate RANK score (MRS) were performed by 2 independent neurologists. The patients received a family follow-up at 1 week, 1 month, and 3 months after the interventional procedure. Neurologic sequelae, intracranial hemorrhages, and deaths were recorded as an endpoint. Follow-up clinical and imageological examinations were scheduled at 6 months after the intervention.
Imageological follow-up and analysis
Follow-up brain CT perfusion or MRA perfusion by 2 imageological scanners was scheduled 6 months after the procedure. Assessment of cerebral perfusion (before and after the procedure) was performed by 2 independent investigators who were blinded to clinical and angiographic outcomes. CT perfusion data were analyzed separately off-line at a workstation by using CT software (GE CT perfusion V4.4.2, software: AW VolumeShare 2) or MRA perfusion software (Simens Skyra, software: NUMARIS/4). Cerebral blood volume (CBV), cerebral blood flow (CBF), time to peak (TTP), and mean transit time (MTT) were calculated. The topographic pattern was categorized into 3 groups: absence of asymmetry, watershed zones, and vascular territory hypoperfusion. A grading system for qualitative assessment of brain perfusion of the region of interest was proposed as follows: 0 = complete perfusion; 1 = hypoperfusion with preserved cerebral blood volume (lower cerebral blood flow, delayed time to peak, increased mean transit time, decreased flow, and normal or elevated cerebral blood volume); and 2 = hypoperfusion without adequate blood volume (decreased cerebral blood volume) (18). Improvement in brain perfusion after the procedure was defined as at least a 1 categorical number decrease in the region of interest according to the grading system.
Statistical analysis
Discrete data are expressed as counts and percentages. Continuous data were presented as mean ± standard deviation (SD). The χ2 or Fisher’s exact test (when the group number was ≤5) was used to compare groups of categorical data. The Wilcoxon-Mann-Whitney U test was applied to compare groups of continuous unpaired data. Paired continuous data were compared by the Wilcoxon signed rank-sum test. Pearson’s correlation coefficients were used to assess the correlation between the change in brain perfusion and the changes in the results of 9 neuropsychological tests. A two-sided probability value <0.05 was considered statistically significant. SPSS 19.0 was used for statistical analyses.
Results
The baseline characteristics and neurocognitive function in Table 1
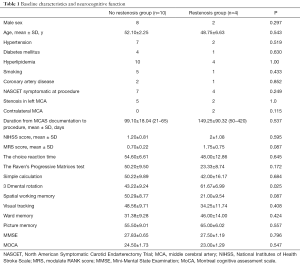
Full table
For chronic MCA stenosis and cerebral ischemia patients, angioplasty is a difficult and controversial therapy, and thus is not performed frequently either in China or internationally. From August 2013 to May 2015, angioplasty was attempted in 14 MCAS patients (9 men; mean ± SD, 50±9.16 years; range, 45–65 years) with objective ipsilateral hemisphere ischemia. Mean duration from the time of documentation of stenosis to the procedure was 120.15±113.05 days (range, 21–420 days). A total of 11 patients (78.5%) had prior ipsilateral ischemic events, 5 patients showed transient ischemic attack (TIA), and 6 patients experienced their last event within 6 months; 3 of the 14 patients did not have a prior ischemic event and were thus “asymptomatic”. Of the three patients, 2 patients complained of dizziness and one complained of limb numbness, with MCA severe stenosis of blood vessels found during routine clinical examination. Meanwhile, 7 patients (50%) had left MCAS >70%, (not entirely occluded), and 2 patients (14%) had contralateral MCAS >70% with recurrent stenosis in the position of stenting after 6 month follow-up.
Results of the angioplasty procedures
Angioplasty technical success was achieved in 14 parents (100%). The 14 patients had been clinically followed up with for 6 months. We found that 10 patients had no recurrent MCAS in the angioplasty site and had a significant improvement in the associated brain perfusion situation and cognitive function as compared before and after angioplasty. There were 4 patients with restenosis in the angioplasty, 1 of whom had significantly improved brain blood perfusion and cognitive function when comparing the status before and 6 months after angioplasty (Figures 1,2). The experimental groups were compared to the clinical groups in terms of whether stenosis recurred.
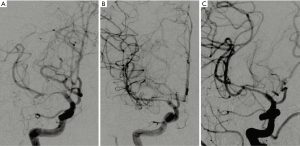
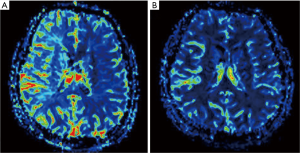
One patient with right MCAS (1 of 14; 7%) received angioplasty and had neurological deficits appear after 2 days. After CT and TCD examination, we found no liability stenosis or occlusion of large blood vessels, but the patient was diagnosed with perforator infarction, and treated in intensive care unit (NICU). The patient left the hospital, and after 18 days the symptoms reappeared, with the examined NIHSS score decreasing from 7 points at appeared neurological to 2 points when left the hospital. At 6 months follow-up, the patient had an NIHSS score of 0 and an MRS score of 0. A recurrent MCAS was found with digital subtraction angiography (DSA) in the angioplasty site; we placed this patient in the restenosis group analysis.
The neurocognitive and neurological functions at baseline and 6 months after the procedure in recurrent stenosis and nonstenosis groups (Table 2)
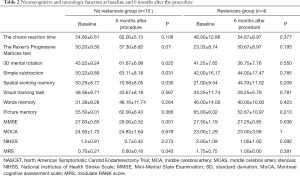
Full table
In the nonstenosis group, we found significant improvements in MMSE (before 27.83±0.65 versus after 29.00±0.52; P=0.001), 3D mental rotation (before 43.22±9.24 versus after 61.67±6.99; P=0.025); simple calculation (before 50.22±9.89 versus after 63.11±8.18; P=0.031), spatial working memory (before 50.29±8.77 versus after 70.58±8.05; P=0.030. In the recurrent stenosis group, we found no statistically significant changes in cognitive function compared with the baseline and after a 6-month follow up.
Discussion
Our research confirms that the brain artery angioplasty treatment is safe and effective in MCAS. The angioplasty was successful in all enrolled 14 patients who underwent this procedure. No new ischemic events occurs with the large vessels after the angioplasty procedure. One patient developed surgerical complications (perforating branches event), and in the 6-month follow-up, no recurrent stroke or death happened in the 14 patients; 4 patients had restenosis, and 2 patients had bilateral MCA stenosis.
This is one of the few studies to examine the correlation of MCA angioplasty and cognitive function improvement. Even though, recurrent stenosis in MCA stenting is a common complication (19), little research has been done concerning the relation of recurrent stenosis and change in cognitive function. In our study, we found a positive correlation between cognitive function and brain perfusion, and significant brain perfusion corresponded to significant improvement in cognitive function. Thus, it can be concluded that MCAS patients can have an improvement in cognitive function and brain perfusion when accepting the MCA angioplasty. In a 6-month follow-up, some patients with recurrent stenosis also showed improvements in brain perfusion and cognitive function. Furthemore, our findings indicated that increased cerebral perfusion was reflected in improved global cognitive function as represented by scores on the MMSE, 3D mental rotation, simple calculation, and spatial working memory. The improvement in cognitive function in the successful group can only be explained by the restoration of cerebral perfusion and improvement of hemisphere ischemia.
Restoration of cerebral circulation may lead to cognitive improvement in certain patients. We know that chronic cerebral hypoperfusion (20,21), critically contributes to cognitive impairment (19) and ischemic events in patients with symptomatic or asymptomatic internal carotid artery system stenosis. We hypothesize that the potential causes are as follows. Firstly, the restored blood flow can increase the brain oxygen supply, facilitate the formation of new collateral circulation, and inhibit lipid peroxidation. Thus, there is an inhibition of the onset and reduction of neuronal death (8). Second, oxygen-free radicals can be effectively removed after blood flow recovery. Free radicals can not only cause the oxidation of unsaturated fatty acids in the meninges but also cause lysosomal damage, aggravating cognitive and psychological functions (22). Third, the surgery itself, as a positive stimulus, can help patients to respond to chronic environmental stresses and have a positive psychological suggestive therapeutic effect (23). Fourth, angioplasty can reduce the chronic inflammatory response of patients. Previous studies have found that inflammation is associated with poor physical and mental health. Chronic low-grade inflammation is closely related to post-stroke major depressive disorder (MDD) (24,25). Other unknown factors may also affect this process.
Several limitiations to our study should be addressed. First, we might have overestimated the impact of cognitive restenosis. Next, the exact time could be measured from the stenosis open, and we did not analyze whether the establishment of collateral circulation and improvement of cognitive function had relevance from then on. Furthermore, the patient’s learning efficiency might have affected the cognitive outcomes of the study. Finally, the sample size was small, and thus the results might not be not stable; an analysis of large amounts of data may yield different results. We will continue to collect patient data and try our best to collect more cases for follow-up reports. However, our study demonstrated that angioplasty surgery can significantly improves neurocognitive function in patients with MCAS and objective cerebral ischemia.
Acknowledgments
Funding: This work was supported by Wu Jieping Medical Foundation to Dr. Zhaohong Kong (number:320.6750.19092-23) and The National Natural Science Foundation grant to Dr. Zhaohui Zhang (number: 81671051).
Footnote
Reporting checklist: The authors have completed the STROBE Reporting Checklist. Available at http://dx.doi.org/10.21037/apm-20-15
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-15
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-15). All authors report grants from Wu Jieping Medical Foundation (number: 320.6750.19092-23), The National Natural Science Foundation (number: 81671051) outside the submitted work.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee of Renmin Hospital, Wuhan University (No. 2019-X-16). We obtained written, informed consent from each patient or substitute decision-maker.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke 1995;26:14-20. [Crossref] [PubMed]
- Liu CY, Chen CQ. Intra-and extracranial atherosclerotic stenosis in China: epidemiology, diagnosis,treatment and risk factors. Eur Rev Med Pharmacol Sci 2014;18:3368-79. [PubMed]
- Huang YN, Gao S, Li SW, et al. Vascular lesions in Chinese patients with transient ischemic attacks. Neurology 1997;48:524-5. [Crossref] [PubMed]
- Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113:555-63. [Crossref] [PubMed]
- Arenillas JF, Molina CA, Montaner J, et al. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke 2001;32:2898-904. [Crossref] [PubMed]
- Kern R, Steinke W, Daffertshofer M, et al. Stroke recurrences in patients with symptomatic vs asymptomatic middle cerebral artery disease. Neurology 2005;65:859-64. [Crossref] [PubMed]
- Kasner SE, Lynn MJ, Chimowitz MI, et al. Warfarin vs aspirin for symptomatic intracranial stenosis: subgroup analyses from WASID. Neurology 2006;67:1275-8. [Crossref] [PubMed]
- Kimura M, Robinson RG, Kosier JT. Treatment of cognitive impairment after poststroke depression. A double-blind treatment trials. Stroke 2000;31:1482-6. [Crossref] [PubMed]
- Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a Balloon-Expandable Intracranial Stent vs Medical Therapy on Risk of Stroke in Patients With Symptomatic.Intracranial Stenosis:the VISSIT Randomized Clinical Trial. JAMA 2015;313:1240-48. [Crossref] [PubMed]
- Kong Z, Jiang J, Deng M, et al. Edaravone reduces depression severity in patients with symptomatic intracranial stenosis and is associated with the serum expression of sex hormones. Medicine (Baltimore) 2020;99:e19316. [Crossref] [PubMed]
- Wind AW, Schellevis FG, Van Staveren G, et al. Limitations of the Mini-Mental State Examination in diagnosing dementia in general practice. Int J Geriatr Psychiatry 1997;12:101-8. [Crossref] [PubMed]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. [Crossref] [PubMed]
- Wei W, Lu H, Zhao H, et al. Gender differences in children's arithmetic performance are accounted for by gender differences in language abilities. Psychological Science 2012;23:320-30. [Crossref] [PubMed]
- Johansen-Berg H, Dawes H, Guy C, et al. Wade and Paul M. Matthews. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 2002;125:2731-42. [Crossref] [PubMed]
- Roca M, Parr A, Thompson R, et al. Executive function and fluid intelligence after frontal lobe lesions. Brain 2010;133:234-47. [Crossref] [PubMed]
- Li Y, Wang EF, Zhang QY. Functional connectivity changes between parietal and prefrontal cortices in primary insomnia patients: evidence from resting-state fMRI. Eur J Med Res 2014;19:32. [Crossref] [PubMed]
- Culham JC, Brandt SA, Cavanagh P, et al. Cortical fMRI Activation Produced by Attentive Tracking of Moving Targets. J Neurophysiol 1998;80:2657-70. [Crossref] [PubMed]
- Zi W, Gong Z, Shuai J. Novel Approaches in Evaluating and Predicting the Potential Benefit of Middle Cerebral Artery Angioplasty & Stenting. J Neuroimaging 2015;25:620-5. [Crossref] [PubMed]
- Bakker FC, Klijn CJM, Jennekens-Schinkel A, et al. Cognitive disorders in patients with occlusive disease of the carotid artery: a systematic review of the literature. J Neurol 2000;247:669-76. [Crossref] [PubMed]
- Klijn CJM, Kappelle LJ, Tulleken CAF, et al. Symptomatic carotid artery occlusion: a reappraisal of hemodynamic factors. Stroke 1997;28:2084-93. [Crossref] [PubMed]
- Grubb RL Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998;280:1055-60. [Crossref] [PubMed]
- Sierra C. Cerebral small vessel disease, cognitive impairment and vascular dementia. Panminerva Med 2012;54:179-88. [PubMed]
- Tamnes CK, Walhovd KB, Engvig A, et al. Regional hippocampal volumes and development predict learning and memory. Dev Neurosci 2014;36:161-74. [Crossref] [PubMed]
- Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr 2018;30:1-16. [Crossref] [PubMed]
- Chen CY, Chen CL, Yang YH, et al. Poststroke Depressive Symptoms Are Associated With Increased Oxidative Deoxyribonucleic Acid Damage. J Neuropsychiatry Clin Neurosci 2018;30:139-44. [Crossref] [PubMed]


