
Pneumonia caused by Talaromyces marneffei in an epidermal growth factor receptor (EGFR) mutation-positive advanced lung adenocarcinoma patient: a case report
IntroductionOther Section
Talaromyces marneffei (T. marneffei, previously known as Penicillium marneffei) is a major fungal pathogen endemic to Southeast Asia. It causes Talaromycosis (formerly known as Penicilliosis), an invasive infection that manifests as either local or disseminated infection, which is mainly dependent on the immune status of the host. As a thermally dimorphic pathogen, colonies of T. marneffei are yellowish-green with diffusing red pigment when incubated at room temperature; however, when incubated at 37 °C, the colonies become white, dry, and yeast-like. Since the discovery of T. marneffei in 1950s, our knowledge of this fungus has been greatly improved, despite still lacking a comprehensive understanding. Certain bamboo rat species have been considered as natural carriers of T. marneffei (1). At present, a sufficient explanation regarding the route of transmission of the disease is unavailable, although infection possibly occurs via skin damage, the digestive tract, or inhalation of conidia.
HIV-infected patients with a cluster of differentiation (CD4) cell count of <100 cells/µL (2) are predominantly susceptible to Talaromycosis, however, cases of T. marneffei infection in non-HIV-infected patients have been increasingly reported in recent years (3). Non-HIV-infected patients are defined as those who are immunodeficient, immunocompromised, or immunocompetent, including primary immunodeficiency (PID) pediatric patients (4), patients with adult-onset immunodeficiency syndrome caused by anti-interferon-gamma (anti-IFN-γ) autoantibodies (5), patients with solid organ and hematopoietic stem cell transplantation, patients with autoimmune diseases, malignant tumors (6-8), or chronic obstructive pulmonary disease (COPD) (9), and even previously healthy patients (10).
There has been no reported case of Talaromyces marneffei infection in targeted therapy patients. The present study reports a case of an advanced epidermal growth factor receptor (EGFR) mutation-positive lung adenocarcinoma patient with pneumonia caused by T. marneffei.
We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2137).
Case presentationOther Section
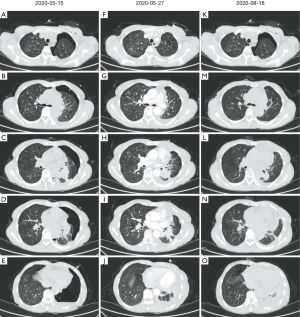
A 59-year-old female with a 3-month history of productive cough and progressive dyspnea attended our hospital for treatment on May 15, 2020. The patient suffered from obvious breathing difficulty, especially during physical labor or in fatigue, but presented without fever, chest pain, palpitations, or weight loss. She denied any recent travel or contact with bamboo rats. From May 3 to May 14, she was treated at a local hospital. Chest computed tomography (CT) scans showed a mass in the left lower lung, multiple plaques and nodules in both lungs, and left pleural effusion (Figure 1). She underwent closed thoracic drainage, and enclosure pneumothorax was developed postoperatively. She was transferred to our hospital for further diagnosis and treatment.
We performed a physical examination of the patient upon admission. Her body temperature was 36.2 °C. The closed thoracic drainage tube in the left front chest was unobstructed and without crackles in both lungs.
During admission, she underwent routine blood, sputum, pleural fluid, urine, and stool laboratory tests. Chest CT scan and bronchoscopy with transbronchial lung biopsy (TBLB) and bronchoalveolar lavage (BAL) were also performed. Her blood was HIV-negative, and she had normal CD4 (905 cells/µL) and cluster of differentiation 8 (CD8) (410 cells/µL) counts. Furthermore, arterial blood gas was analyzed (FiO2 =0.21), and the results were as follows: pH of 7.44, partial pressure of oxygen (PO2) of 86 mmHg, partial pressure of carbon dioxide (pCO2) of 41 mmHg, standard bicarbonate (HCO3-std) of 27.5 mmol/L, oxygen saturation (SaO2) of 97%, and oxygenation index of 409. Moreover, the patient’s white blood cell count was normal [total count: 6.14×109/L (3.5–9.5×109/L), neutrophil count: 3.41×109/L (1.8–6.3×109/L), neutrophils: 55.6% (40–75%), lymphocytes count: 2.09×109/L (1.1–3.2×109/L), lymphocytes: 34% (20–50%)]. Also, hemoglobin was 122 mg/L (115–150 mg/L), platelets were 314×109/L (125–350×109/L), C-reactive protein (CRP) was <7.2 mg/L (0–8 mg/L), erythrocyte sedimentation rate (ESR) was 5 mm/h (<15 mm/h), and procalcitonin (PCT) was normal (<0.05 ng/mL). The 1,3-β-D glucan (G) and galactomannan (GM) tests of serum, G test of bronchoalveolar lavage fluid (BALF), and the acid-fast bacillus test of the sputum and BALF were all negative. Pleural fluid fungi and bacterial cultures were also negative. Moreover, blood spartate aminotransferase, blood alanine aminotransferase, blood creatinine, blood urea nitrogen, blood sugar, and serum electrolytes were all normal.
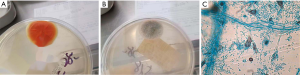
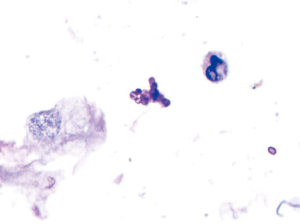
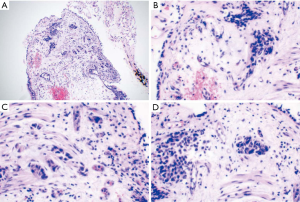
The GM test of BALF was positive. One yellowish-green colony with diffusing red pigment was detected in the patient’s sputum sample by fungi culture incubated at room temperature (Figure 2). BALF cytology examination identified some periodic acid-Schiff (PAS)- and periodic acid methenamine silver (PAM)-positive yeast-like organisms with a central septum (Figure 3). Histological examination (HE) of the left lung lesion by TBLB indicated that the patient had non-small cell lung cancer (NSCLC) with a possibility of transforming into adenocarcinoma (Figure 4). Immunohistochemistry revealed that antigen Ki-67 (15%), thyroid transcription factor 1 (TTF-1) (+++), and Napsin A (+++) were positive, while programmed death-ligand 1 (PD-L1) was negative. Next generation sequencing (NGS) detection of the biopsy tissues by TBLB showed EGFR p.Glu746_Ala750del(E19). Heterosexual cells were detected in the pleural fluid.
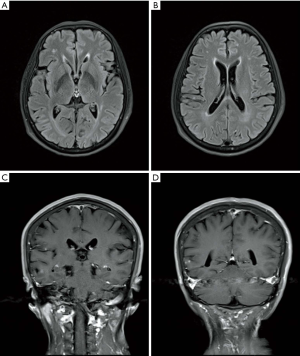
Chest CT revealed a mass in the left lower lung, multiple nodes in both lungs, and hydropneumothorax (Figure 1A,B,C,D,E). Brain metastasis was observed by contrast material-enhanced magnetic resonance imaging (MRI) of the brain (Figure 5). No abnormalities of the upper abdomen were found by contrast material-enhanced CT or whole body bone emission CT (ECT). Based on the above results and observations, the patient was diagnosed with a T. marneffei lung infection and EGFR mutation-positive stage IV lung adenocarcinoma (T4N3M1c).
Following admission, a pressure-attracting implement was connected to a closed thoracic drainage tube to prevent air leakage. After being diagnosed with a T. marneffei infection, the patient received intravenous liposomal amphotericin B as an anti-fungal treatment followed by oral icotinib as an anti-tumor treatment. The patient’s conditions improved after 2 weeks of treatment, and she was prescribed oral itraconazole and icotinib therapy and discharged. The initial dose of liposomal amphotericin B was 5 mg/day daily, increased by 5 to 40 mg/day. However, when the dose was increased to 50 mg/day, the patient developed hypokalemia, which is a common side effect of liposomal amphotericin B. Intravenous dexamethasone was used for pretreatment prior to each intravenous injection of liposomal amphotericin B.
Following treatment, CT re-examination showed that the mass was significantly absorbed and most lung nodules disappeared, however, pleural effusion was unchanged (Figure 1). Until Nov 5, 2020, follow-up showed no relapse of T. marneffei infection or tumor progression. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion and conclusionsOther Section
The present study reports a local infection case of Talaromycosis in a non-HIV-infected patient with advanced lung adenocarcinoma. The patient had normal CD4 and CD8 counts, which suggested normal cell immunity and explained why the infection was not disseminated. Our literature review identified few cases of lung cancer with Talaromycosis (8,11). Lung cancer will impair the immune system, but whether this is an important cause of Talaromycosis remains to be confirmed by future studies. Although the patient denied any recent travel or contact with bamboo rats, T. marneffei could possibly exist in some environmental reservoirs, such as soil (1).
Clinical manifestations of T. marneffei infection vary, and its severity depends on the immune status of patient, as well as the time of diagnosis and treatment. Disseminated infections are usually associated with immunodeficient patients, including patients with HIV/AIDS, PIDs, or impaired cellular immunity. The symptoms and signs of disseminated T. marneffei infection are both systemic and local. In addition to fever, weight loss, and fatigue, depending on the organ systems invaded by the fungus, the specific symptoms and signs include erythra or skin lesions, hepatosplenomegaly, lymphadenopathy, blurred vision, redness and foreign body sensation, and respiratory and gastrointestinal abnormalities. Cases of local infection cases typically exhibit localized symptoms and signs, and rarely involve systemic symptoms.
The patient in the current study presented with fatigue, coughing and sputum production, exertional dyspnea, and progressive dyspnea mainly due to growing pleural effusion, but did not have a fever, which is also common in infectious diseases. Coughing is not specific to a certain lung disease, as it could have been caused by both lung cancer and/or infectious lung disease in the current case. However, sputum production is commonly observed in obstructive pneumonia or complicated infectious lung diseases caused by lung cancer.
Mycological culture of body fluids or tissues is a highly effective method for the diagnosis of T. marneffei infection. Bone marrow, skin biopsy, and blood are the three samples with the highest sensitivity in the disseminated infection (12). The current case is a local infection in the lung, and T. marneffei was identified based on the typical morphology of colonies in the sputum samples cultured and the microscopic morphology of the BALF cytology examination. Therefore, T. marneffei was diagnosed as the cause of the patient’s pneumonia.
At the time of admission, there were multiple nodules in the patient’s lungs, some of which were solid and others that were ground-glass on the chest CT film. However, whether these nodules were metastatic cancer nodules or infected lesions was difficult to confirm. According to the CT findings of the chest during the follow-up (Figure 1F,G,H,I,J,K,L,M,N,O), most of the nodules had disappeared within a short period of time following anti-infective treatment of infected lesions. We treated this patient according to the first-line treatment plan for HIV patients with Talaromycosis, but considering the low body mass index (BMI) of the patient (18.55 kg/m2) and drug side effects, we did not utilize a sufficient dose of liposomal amphotericin B. However, as can be seen from the treatment results, the anti-infective therapy was effective, and our patient's symptoms improved significantly.
Ositinib is an optimal medication for treating advanced EGFR mutation-positive lung adenocarcinoma patients with brain metastasis. However, in this case, icotinib was prescribed to the patient due to medical insurance policy and economic considerations. The use of targeted drugs prevents the potential spread of infection caused by chemotherapy. Moreover, simultaneous targeted therapy and anti-infective therapy were found to be safe in this case, which may have been one of the factors leading to the successful treatment of the patient, coupled with the timely diagnosis and treatment of the T. marneffei infection.
In summary, T. marneffei infection is not limited to HIV-positive patients, especially those who reside in endemic regions. Microbiological examination of sputum, BALF, and pleural fluid play an important role in the diagnosis of patients with malignancies, despite few clinical manifestations of infection. Furthermore, missed diagnosis or misdiagnosis of the infection might result in the dissemination of pathogenic microorganisms, especially for those patients who require chemotherapy, radiotherapy, or immunotherapy.
AcknowledgmentsOther Section
Funding: This work was supported by grants from the Foundation of Priority Project of Science and Technology Plan of Fujian Province (No. 2014Y0037), and the Clinical Key Specialty Construction Project of Fujian Province (No. 2015-593 and No. 2017YZ0001-2).
FootnoteOther Section
Reporting Checklist: We present the following article in accordance with the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2137
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2137). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Tsang CC, Lau SKP, Woo PCY. Sixty Years from Segretain's Description: What Have We Learned and Should Learn About the Basic Mycology of Talaromyces marneffei? Mycopathologia 2019;184:721-9. [Crossref] [PubMed]
- Cao C, Xi L, Chaturvedi V. Talaromycosis (Penicilliosis) Due to Talaromyces (Penicillium) marneffei: Insights into the Clinical Trends of a Major Fungal Disease 60 Years After the Discovery of the Pathogen. Mycopathologia 2019;184:709-20. [Crossref] [PubMed]
- Chan JF, Lau SK, Yuen KY, et al. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect 2016;5:e19. [Crossref] [PubMed]
- Guo J, Li BK, Li TM, et al. Characteristics and Prognosis of Talaromyces marneffei Infection in Non-HIV-Infected Children in Southern China. Mycopathologia 2019;184:735-45. [Crossref] [PubMed]
- Lee PP, Mao H, Yang W, et al. Penicillium marneffei infection and impaired IFN-gamma immunity in humans with autosomal-dominant gain-of-phosphorylation STAT1 mutations. J Allergy Clin Immunol 2014;133:894-6.e5. [Crossref] [PubMed]
- Atalay A, Koc AN, Akyol G, et al. Pulmonary infection caused by Talaromyces purpurogenus in a patient with multiple myeloma. Infez Med 2016;24:153-7. [PubMed]
- Zhang J, Huang X, Zhang X, et al. Coinfection of disseminated Talaromyces marneffei and Mycobacteria kansasii in a patient with papillary thyroid cancer: A case report. Medicine (Baltimore) 2017;96:e9072. [Crossref] [PubMed]
- Lin F, Qiu Y, Zeng W, et al. Talaromyces marneffei infection in a lung cancer patient: a rare case report. BMC Infect Dis 2019;19:336. [Crossref] [PubMed]
- De Monte A, Risso K, Normand AC, et al. Chronic pulmonary penicilliosis due to Penicillium marneffei: late presentation in a french traveler. J Travel Med 2014;21:292-4. [Crossref] [PubMed]
- Shuangshuang H, Haiyan L, Junru Y, et al. Penicilliosis marneffei of the lung in immunocompetent patients: three cases report and literature review. Chinese Journal of Clinical Infectious Diseases 2018;11:51-5,60.
- Peng Y, Ping L, Qiu-wen L, et al. Talaromyces marneffei infection complications in an HIV-negative patient with lung cancer: a case report and review of literature. Electronic Journal of Emerging Infectious Diseases 2019;4:169-72.
- Supparatpinyo K, Khamwan C, Baosoung V, et al. Disseminated Penicillium marneffei infection in southeast Asia. Lancet 1994;344:110-3. [Crossref] [PubMed]


