
Effectiveness of using hydrocolloid dressing combined with 3M Cavilon No-Sting Barrier Film to prevent facial pressure injury on medical staff in a COVID-19 designated hospital in China: a self-controlled study
Introduction
Since December 2019, there has been a series of unexplained cases of pneumonia reported in Wuhan, China (1). In late January 2020, the COVID-19 outbreak was rapidly transmitted arousing enormous global concern (2). On 30 January, 2020, the World Health Organization (WHO) declared that the outbreak of 2019-nCoV constituted a public health emergency of international concern (PHEIC) (3).
Although COVID-19 can be transmitted through contact, respiratory droplet transmission is the main route of transmission (4). By 10 April 2020, human-to-human transmission of COVID-19 had infected 1,540,000 people in 211 countries.
Medical staff who care for COVID-19 patients have to wear personal protective equipment (PPE) including N95 masks, eye protection, gowns, and gloves (5). Due to the external environment and a shortage of supplies, medical personnel often wear PPE continuously for 4 to 8 hours or more. Wearing medical protective equipment in a hot and humid environment for long periods of time is known to cause pressure injuries to the nose and face.
The 2016 National Pressure Ulcer Advisory Panel (NPUAP) revised the definition and stages of pressure injury, with the revised staging system using the term “injury” instead of ulcer and including pressure injuries associated with medical devices. Medical device-related pressure injuries (MDRPIs) result from the use of devices designed and applied for diagnostic or therapeutic purposes. They occur because of the constant pressure on the skin caused by the device or the apparatus used to fix the device. The resultant pressure injury generally conforms to the pattern or shape of the device (6).
People exposed to long-term pressure caused by N95 masks are prone to skin and subcutaneous soft tissue damage, namely compression damage. This is most common on the bridge of the nose and the two sides of the nose wing. Prolonged wearing of a mask results in continuous pressure on the local skin, impaired blood circulation, nutrient deficiency in tissues, and increased sweating caused by the relatively confined space within protective clothing, all of which promote facial pressure injuries. Protecting medical staff by preventing the occurrence of facial pressure injury has become an important issue that needs to be urgently addressed.
There is clear evidence that indicates that prophylactic use of multi-layer foam dressings as a part of standard prevention measures is beneficial (7). However, foam dressings are of a certain thickness, which affects the tightness of the PPE. A meta-analysis of randomized controlled trials showed that using a hydrocolloid dressing significantly decreased the incidence of facial pressure injury caused by noninvasive ventilation (8). However, after the use of hydrocolloid dressing, some medical personnel still suffered pressure injuries. In a bench model, a liquid barrier film provided a greater reduction in the coefficient of friction against the skin. In the context of preventative use on unwounded skin, applying a liquid barrier film may be more effective than applying a silicone dressing in reducing the risk of pressure ulcers (9). Therefore, this study aimed to investigate the effect of hydrocolloid dressing combined with 3M Cavilon No-Sting Barrier Film on the prevention of pressure ulcers in medical staff in a COVID-19 designated hospital.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1615).
Methods
Study design
This self-controlled study was conducted in the isolation ward of the 2nd Affiliated Hospital of Wenzhou Medical University, Zhejiang province, China. It is divided into two phases according to the time that medical workers stayed in the isolation ward. The first two weeks of medical personnel entering the isolation ward is phase I (6th January to 19th January 2020) and the second two week is phase II (20th January to 2nd February 2020). During phase I, Medical personnel used hydrocolloid dressings on their face before wearing isolation protective equipment. During phase II, medical personnel sprayed 3M Cavilon No-Sting Barrier Film on the face before applying hydrocolloid dressing. The rest of the steps were consistent with phase I.
Participants
A total of 116 medical workers were selected as research subjects from 6th January to 2nd February 2020. All the workers were using medical protective masks, goggles and conjoined protective clothing for protection, nasal and facial skin before using isolation protective equipment was complete, were working more than 4 hours a day, and were working in the isolation ward for 4 weeks. All subjects provided informed consent and volunteered to participate in the study. Medical personnel with any of the followings were excluded from the study: allergy to 3M Cavilon No-Sting Barrier Film or hydrocolloid dressing, or had dermatopathy a history. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Ethics Committee of the 2nd Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (No. L-2020-24).
Standard preventive care and intervention
Medical personnel were trained in the use of PPE. During phase I, Medical personnel were trained to gently scrub their face with warm water and allow it to dry before wearing isolation protective equipment. All hydrocolloid dressings were cut using the same standard procedure: in a circular way according to the shape and size of the facial contour. The preventive intervention included pasting hydrocolloid dressing over the nasal bridge, cheekbones and forehead as shown in the Figure 1. A tension-free paste method was adopted to apply the hydrocolloid dressing to the nose, face and other easily compressed parts, meaning the dressing was placed flat when pasted, then pressure was applied with the fingers from the center of the dressing to the surrounding area with care to avoid creating tension on the dressing.
Personal isolation protective equipment was worn according to the prevention and control standard of COVID-19. Hair was neatly tied into a ball or fixed so that the top band of the mask is fixed on the head of the ball in a female. After washing hands carefully, the N95 respirator was put on and the nose clip pressed tightly, and the tightness tested. Then goggles, a disposable hat, latex gloves, and protective shoe covers were put on in turn. If contamination or damage was discovered, personal protective equipment was replaced (10). Medical protective equipment was removed in reverse order after work. Finally, the hydrocolloid dressing was removed at zero angle while the skin was fixed in the opposite direction with fingers. This is a tension-free way to remove the hydrocolloid dressing. The occurrence and staging of the pressure injury were recorded by the ward supervisor during each shift.
During phase II, medical personnel sprayed 3M Cavilon No-Sting Barrier Film on the face before applying hydrocolloid dressing. The area covered by the 3M Cavilon No-Sting Barrier Film was the nasal bridge, cheekbones and forehead, at least 0.5 centimeters beyond that of the hydrocolloid dressing. It was then gently massaged with the fingers into the skin after it was dry. The rest of the steps were consistent with phase I.
Outcomes and their measurement
The primary outcome was facial temperature of the medical workers after removing the hydrocolloid dressing. Facial temperature was defined as the average temperature of skin on the forehead and cheeks, which was measured and recorded daily by the ward supervisor with an infrared thermometer. The secondary outcome was the rate of any grade of pressure injury detected in the face area. Lesions were classified according to the NPUAP classification. Pressure injury stage 1: erythema with no whitening in response to finger pressure, skin integrity in tact; stage 2: partial cortical loss with dermal exposure; stage 3: total skin loss; stage 4: total skin and tissue loss. The occurrence and staging of the pressure injury were recorded by the ward supervisor who monitored the state of face skin after each study phase.
Another outcome was skin comfort level. A self-made facial comfort questionnaire was adopted. There are 5 items: pressure pain sensation, humidity sensation, itching sensation, skin burning sensation, and self-tolerance level. Each item was rated from 0 to 3 points. The total score ranges from 0 to 15 points. A total score of less than 7 points is defined as good comfort level. On the contrary, it is general comfort level. The skin comfort level was assessed by themselves once a fortnight.
Statistics
All statistical analyses were performed using the Statistical Package of Social Sciences (SPSS) version 19.0. Descriptive statistics were used to describe the data and to extract the tables and charts. Chi-square tests, ANOVA with repeated measures were used for interpreting the data in inferential statistics. P<0.05 was considered to indicate a statistically significant difference.
Results
Among the 116 medical personnel, 42 were males and 74 were females, aged from 26 to 40 years, with an average age of 33.72±2.76 years. The average age was 33.32±2.57 years for women and 34.4±2.98 years for men. The body mass index is 21.45±1.61 kg/m2 for females and 22.72±1.33 kg/m2 for males.
Analysis of variance with repeated measures on the facial temperature indicator between the two phases shows that from the baseline (day 1) to the end of the study (day 14) the average facial local temperature during phase I was higher than phase II. However there was no statistically significant difference (P>0.05, Figure 2).
In this study, the stages of pressure injury of medical staff were all stage 1. Analysis of the data showed that phase II showed statistically significant differences in terms of the incidence of pressure injury by intervention (P<0.05). This means that hydrocolloid dressing combined with 3M Cavilon No-Sting Barrier Film could reduce the occurrence of pressure injury (Table 1).

Full table
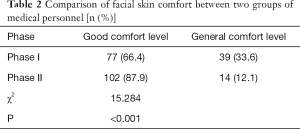
A total score of less than 7 points is defined as good comfort level. Seventy-seven staffs reported a good comfort level during phase I, while 102 staff reported a good comfort level during phase II. The result showed that the facial skin comfort of medical staff was higher in Phase II with a statistically significant difference (P<0.05, Table 2).
Full table
Discussion
Medical staff are required to wear protective equipment continuously while working in the isolation wards, which increases stress and intensity (11). As well as mental stress, the heavy protective measures add physical stress. Medical personnel working in isolation wards needed to wear tightly fixed medical protective masks and goggles for long periods of time, which increases the pressure on the local skin, and the prevalence of cutaneous irritation associated with N95 mask and goggle use is high (12). This can cause pressure ulcers, a MDRPI. Safety of health-care workers must always be ensured (13), and protecting front-line medical workers from MDRPI under high intensity work pressure is an urgent and global issue.
A review about preventing and treating pressure ulcers mentioned that one of the top 10 priorities includes using different types of protectors in preventing pressure ulcers (14). The effectiveness of silicone bordered dressings supports their use to assist sacral pressure ulcer prevention in patients at high risk of pressure ulcers (15). But silicone bordered dressings have a certain thickness which will affect the tightness of the mask and it is not easy to cut appropriately. Prophylactic dressings can help in the prevention of pressure ulcers, shear and friction damage (16). A study shows there is no difference between using hydrocolloid dressings plus conventional care and using only conventional care in preventing the onset of pressure ulcers in adult patients hospitalized with high risk of developing these events (17). For preventative use to reduce the risk of pressure injury, applying a liquid barrier film may reduce friction better than a silicone dressing (9).
Our study indicated that combination of 3M Cavilon No-Sting Barrier Film, a non-medicated product which acts as a physical barrier on the skin against friction and contamination (18), and hydrocolloid dressing, reduced the incidence of facial pressure injury. The protective coating of 3M Cavilon No-Sting Barrier Film prevents excessive loss of water on the skin surface (preventing cells from dehydrating due to sweat and irritation), which helps to maintain proper elasticity of the skin, thus speeding up the healing of the affected area. The incidence of pressure injury during phase II was significantly lower than in phase I.
The application of a barrier film creating a skin-protective polymer layer dressing is associated with skin integrity issues (19). Increasing skin temperature can be used as a quantitative measurement to predict the development of pressure ulcers and superficial skin changes, and to evaluate support surface capability against microclimate factors (20). In this study there was no statistically significant difference in facial temperature in either phase from baseline (day 1) to the end of the study (day 12). This result was most likely due to our small sample size and selection of only one time to take temperature.
An advantage of 3M Cavilon No-Sting Barrier Film is that it forms a protective film that help to maintain the normal breathing function of local skin. Another benefit is that there is no need to remove the product, reducing the risk of damage to the fragile skin being treated. Our results showed that the facial skin comfort of medical staff improved with a statistically significant difference. The 3M barrier film may be helpful against dermatitis associated pruritus (18). A systematic review show that liquid dressing has significant benefits in terms of pain control and patient comfort because liquid film-forming acrylate has a significant impact on the skin integrity (21), which is consistent with the results of this study. Hydrocolloid dressing combined with 3M Cavilon No-Sting Barrier Film can improve the comfort level.
There were limitations in this self-controlled study. Damage in phase I had an impact on the results of the phase II. The duration of this study was relatively short and could not truly reflect the rate of facial pressure injury. Further research would be required to find an appropriate way to avoid pressure injury.
Conclusions
Hydrocolloid dressing combined with 3M Cavilon No-Sting Barrier Film for facial skin care can effectively reduce the incidence of facial pressure injury in the prevention and control of COVID-19 among medical staff, and can improve the staff skin comfort level while ensuring the isolation and protection effect. Therefore, it is worth further discussion.
Applying research to occupational health practice
Medical personnel spray 3M Cavilon No-Sting Barrier Film on the face. Then they apply hydrocolloid dressing over the nasal bridge, cheekbones and forehead. Finally, they wear personal isolation protective equipment according to the prevention and control standard of COVID-19.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1615
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1615
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1615). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Ethics Committee of the 2nd Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (No.L-2020-24). All subjects provided informed consent and volunteered to participate in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sun P, Lu X, Xu C, et al. Understanding of COVID-19 based on current evidence. J Med Virol 2020;92:548-51. [Crossref] [PubMed]
- Li W, Yang Y, Liu ZH, et al. Progression of Mental Health Services during the COVID-19 Outbreak in China. Int J Biol Sci 2020;16:1732-8. [Crossref] [PubMed]
- WHO. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). World Health Organization. 2020. Available online: https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
- Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020;7:4. [Crossref] [PubMed]
- Wong J, Goh QY, Tan Z, et al. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth 2020;67:732-45. [Crossref] [PubMed]
- Edsberg LE, Black JM, Goldberg M, et al. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System: Revised Pressure Injury Staging System. J Wound Ostomy Continence Nurs 2016;43:585-97. [Crossref] [PubMed]
- Davies P. Role of multi-layer foam dressings with Safetac in the prevention of pressure ulcers: a review of the clinical and scientific data. J Wound Care 2016;25:S4-23. [PubMed]
- Cai JY, Zha ML, Chen HL. Use of a Hydrocolloid Dressing in the Prevention of Device-related Pressure Ulcers During Noninvasive Ventilation: A Meta-analysis of Randomized Controlled Trials. Wound Manag Prev 2019;65:30-8. [Crossref] [PubMed]
- Bernatchez SF, Mengistu GE, Ekholm BP, et al. Reducing Friction on Skin at Risk: The Use of 3M(™) Cavilon(™) No Sting Barrier Film. Adv Wound Care (New Rochelle) 2015;4:705-10. [Crossref] [PubMed]
- Huh S. How to train the health personnel for protecting themselves from novel coronavirus (COVID-19) infection during their patient or suspected case care. J Educ Eval Health Prof 2020;17:10. [Crossref] [PubMed]
- Xiao H, Zhang Y, Kong D, et al. The Effects of Social Support on Sleep Quality of Medical Staff Treating Patients with Coronavirus Disease 2019 (COVID-19) in January and February 2020 in China. Med Sci Monit 2020;26:e923549. [Crossref] [PubMed]
- Kantor J. Behavioral considerations and impact on personal protective equipment use: Early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol 2020;82:1087-8. [Crossref] [PubMed]
- Zhang L, Wang S, Shen J, et al. The mental health of Chinese healthcare staff in non-epicenter of COVID-19: a cross-sectional study. Ann Palliat Med 2020;9:4127-36. [Crossref] [PubMed]
- Chapman S. Preventing and treating pressure ulcers: evidence review. Br J Community Nurs 2017;22 Suppl 3:S37-S40. [Crossref] [PubMed]
- Black J, Clark M, Dealey C, et al. Dressings as an adjunct to pressure ulcer prevention: consensus panel recommendations. Int Wound J 2015;12:484-8. [Crossref] [PubMed]
- Cornish L. The use of prophylactic dressings in the prevention of pressure ulcers: a literature review. Br J Community Nurs 2017;22:S26-S32. [Crossref] [PubMed]
- Cortés OL, Salazar-Beltrán LD, Rojas-Castañeda YA, et al. Use of Hydrocolloid Dressings in Preventing Pressure Ulcers in High-risk Patients: a Retrospective Cohort. Invest Educ Enferm 2018;36:e11. [Crossref] [PubMed]
- Shaw SZ, Nien HH, Wu CJ, et al. 3M Cavilon No-Sting Barrier Film or topical corticosteroid (mometasone furoate) for protection against radiation dermatitis: A clinical trial. J Formos Med Assoc 2015;114:407-14. [Crossref] [PubMed]
- Pivkina AI, Gusarov VG, Blot SI, et al. Effect of an acrylic terpolymer barrier film beneath transparent catheter dressings on skin integrity, risk of dressing disruption, catheter colonisation and infection. Intensive Crit Care Nurs 2018;46:17-23. [Crossref] [PubMed]
- Yusuf S, Okuwa M, Shigeta Y, et al. Microclimate and development of pressure ulcers and superficial skin changes. Int Wound J 2015;12:40-6. [Crossref] [PubMed]
- Schuren J, Becker A, Sibbald RG. A liquid film-forming acrylate for peri-wound protection: a systematic review and meta-analysis (3M Cavilon no-sting barrier film). Int Wound J 2005;2:230-8. [Crossref] [PubMed]




