
Risk factors of pleural effusion after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in late-stage and recurrent ovarian cancer
Introduction
Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC), which prolong the duration of tumor-free survival and overall survival (OS), have been shown to be effective in the treatment of late-stage and recurrent ovarian cancer (1-3). Based on the consensus established by Chinese gynecologic oncologists, this treatment paradigm has been adopted by an increasing number of gynecologists. However, despite the availability of experimental and clinical data, there is ongoing debate regarding this combination treatment. A number of clinical trials have been suspended owing to the occurrence of several serious complications, hindering the promotion and development of this therapeutic approach (4). Physicians should focus on the prevention and treatment of complications occurring after CRS + HIPEC to facilitate the promotion and development of this treatment.
The recent clinical trials focusing on the complications associated with CRS + HIPEC have only summarized the category and prevalence of complications. In a systematic review, Chua et al. reported that the incidence of grade III and IV adverse events during treatment with CRS + HIPEC in patients with late-stage and recurrent ovarian cancer was 0–40% and 0–15%, respectively. The main postoperative adverse events include intestinal obstruction, intestinal fistula, hemorrhage, wound infection, and pleural effusion (PE) (5). The event of PE is one of the most common complications (6-8); however, there have not yet been any studies revealing the risk factors of postoperative PE.
The impact of PE can severely affect the function of the respiratory and circulatory systems. It is most commonly observed in surgical and medical clinical practice. Some studies have reported that the occurrence of PE is a precursor to poor prognosis in the practice of cardiac and liver surgery (9,10). Thus far, the exact reasons responsible for the occurrence of PE remain unknown. This study investigated the risk factors of postoperative PE after CRS + HIPEC in patients with ovarian cancer, with the aim to obtain more guidance regarding its diagnosis and treatment, and improve prognosis.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2334).
Methods
Participants
The participants (stage III–IV or recurrent ovarian cancer) were retrospectively analyzed according to the International Federation of Gynecology and Obstetrics (FIGO) staging system from March 2014 to April 2018 in the Department of Peritoneal Surgery and Gynecology at Shijitan Hospital (Beijing, China). All participants underwent standard CRS + HIPEC and imaging examination within 7 days after surgery (3). They were classified into two groups: PE and non-PE.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Retrospective analysis of data was approved by the local ethic committee. Only data were analyzed, all participant records/information was anonymized and de-identified prior to analysis. Individual consent for this retrospective analysis was waived.
Diagnosis standard
Postoperative PE was diagnosed through chest image examination, and any detected PE was considered a positive result. Classification of the severity of PE was based on the Clavien-Dindo complications grading system (11). The corresponding treatment was applied to symptomatic participants. Albumin and/or diuretics were initially administered, followed by thoracic puncture of participants in whom the effect of the treatment was unsatisfactory. The diagnosis of preoperative and postoperative PE was reached in the same manner.
Observation indicators
The observation indicators included age, body mass index, the pathological type of ovarian cancer, complications (preoperative PE), surgery (i.e., duration, amount of bleeding, involvement of the intestines, involvement of the diaphragm), and laboratory examinations (i.e., plasma albumin and fibrinogen levels before and after surgery).
Statistical analysis
The data were analyzed using the statistical software SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The count data of the clinical index for the two groups were represented by “case” and analyzed using the chi-squared test (χ2) test. The measurement data were analyzed using the rank-sum test, and the multivariate analysis was performed through logistic regression analysis.
Results
Basic information of participants
A total of 77 participants received CRS + HIPEC, including 27 cases of primary and 50 cases of recurrent ovarian cancer. The pathological staging was serous and non-serous carcinoma in 63 and 14 participants, respectively. The median age was 57 years (35–75 years), and 60 participants (77.9%) achieved satisfactory tumor cytoreduction, namely CC0-1. Partial bowel resection and anastomosis were performed in cases with intestinal involvement, while partial diaphragmatic resection was performed in cases with diaphragm involvement. During the perioperative period, 2 participants died; the deaths occurred on days 20 and 26 after surgery due to multi-organ failure and acute renal failure, respectively.
Incidence of PE
The incidence of preoperative PE was 24% (19/77 patients), and the grading of PE was grade I. The incidence of postoperative PE was 57.1% (44/77 patients), including 42.8% and 14.3% with grade I–II and III–IV, respectively. The 11 participants with grade III–IV PE were eventually linked to good prognosis, and underwent thoracic drainage (curation time: 1–15 days).
Patient demographics and general factors
The PE and non-PE groups included 44 and 33 patients, respectively. We compared 8 risk factors between the two groups (Table 1).

Full table
There were statistically significant differences in operative time, intraoperative blood loss, and postoperative level of albumin between the two groups (P<0.05). However, there were no statistically significant differences in age, BMI, preoperative level of albumin, and fibrinogen before and after surgery (P>0.05).
Comparison of disease indicators among patients
Diaphragmatic involvement and preoperative PE were significantly different between the two groups (Table 2).
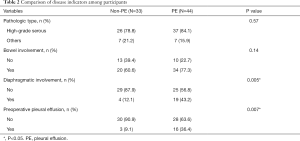
Full table
Univariate and multivariate risk factor analyses for postoperative PE
Logistic regression was used for the multivariate analysis using variables that were statistically significant in the univariate analysis. Preoperative PE and diaphragmatic involvement were statistically significant and identified as independent factors of postoperative PE (P<0.05) (Table 3).
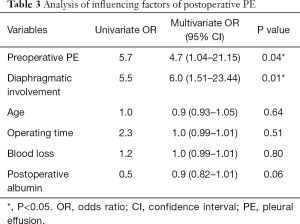
Full table
Risk factor analysis for postoperative PE
The incidence of postoperative PE in participants with preoperative PE and diaphragm involvement during surgery was 100% (Table 4). All 9 participants with these two risk factors developed PE. A total of 8 cases had stage III–IV PE and underwent thoracic puncture.
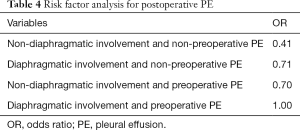
Full table
Discussion
Our research revealed the incidence and risk factors of postoperative PE after CRS + HIPEC in patients with late-stage or recurrent ovarian cancer. The incidence was 57.1%, with 42.8% and 14.3% of patients developing grade I–II and III–IV PE, respectively. These findings are similar to those reported in previous studies. In a retrospective study, Deraco et al. demonstrated that the incidence of grade III–V complications after CRS + HIPE was 26.3%, and the incidence of PE was 13.3% (grade III) (4). Di Giorgio et al. analyzed 511 participants, showing that the incidence of grade III–IV complications was 17.6% (12). The risk factors identified in that study were CC0 and the number of blood transfusions.
The clinical symptom PE is characterized by the accumulation of pathological fluid in the pleural cavity, with manifestation of chest tightness and dyspnea. The function of the respiratory and circulatory systems of patients is severely affected. Its occurrence prolongs hospital stay, affects prognosis, and may be life-threatening to patients. Cascales Campos et al. found that after liver surgery, the development of PE was associated with disease recurrence and poor prognosis (8).
We also found that preoperative PE is an independent risk factor of postoperative PE. The prevalence of preoperative PE was 24%. The participants were asymptomatic, and the presence of pulmonary infection was ruled out. The mechanism of PE was considered to be neoplastic. It is suggested that tumor metastasis to the pleura destroys the integrity of the pleural cavity and blocks the lymphatic vessels, causing the increment in the formation of pleural fluid and reduction of reabsorption (13). Mironov et al. found that patients with late-stage ovarian cancer and positive for PE identified through preoperative computed tomography are linked to poor prognosis (14). In the process of hyperthermic perfusion chemotherapy, the imbalanced function of the pleura is further impaired by the influence of the perfusion pressure and reabsorption of the perfusate, causing an increase in the volume of PE (15).
Another independent risk factor of postoperative PE is partial diaphragmatic resection as the result of diaphragmatic involvement. The CRS + HIPEC treatment involves multiple organ resections, including bowel resection which may lead to the formation of an intestinal fistula. However, contrary to previous speculation, bowel resection, duration of operation, and the amount of bleeding are not related to postoperative PE. The observed increase in the incidence of PE with diaphragmatic involvement may be attributed to the dependency of the filtration and reabsorption of pleural fluid on the integrity of the pleural cavity. Moreover, the main sites of reabsorption of pleural fluid are the lower thoracic mediastinum pleura and septum, which would be destroyed following diaphragmatic resection (16), thus reducing the speed of reabsorption of fluids and causing PE. Moreover, inflammation owing to surgical stimulation also increases the amount of pleural fluids (17). Ashikhmina et al. concluded that in cardiac surgery, the surgical approach is related to the presence of PE (10). Fanfani et al. found that complications of PE increased after surgical resection of subdiaphragmatic lesions in the upper abdomen (18).
The level of plasma albumin in patients with postoperative PE was lower than that measured in the group of non-postoperative PE (P<0.01); however, the difference in the multivariate analysis was not statistically significant. It is established that hypoproteinemia may result in PE by reducing plasma colloid osmotic pressure and allowing a large amount of intravascular fluid to penetrate into the interstitial space. After CRS + HIPEC, patients are more prone to developing hypoproteinemia due to the following factors: (I) increase in blood loss: long duration of surgery is associated with bleeding and loss of protein, and the amount of undetected blood loss can reach 100 mL per operation hour (18); (II) reduced production: patients with intestinal resection, ostomy, and accretion lysis are required to fast for 1 week after the operation, which means reduced absorption of protein; (III) increase in demand: the patients are under stress following surgery, their basal metabolism is significantly increased and the energy demand for recovery also increases; and (IV) in some cases with postoperative abdominal cavity infection, endotoxins stimulate Kupffer cells to release tumor necrosis factor, interleukin (IL)-1, and IL-6, which inhibit the expression of albumin mRNA in liver cells. Hypoproteinemia eventually develops as the result of poor response to exogenous nutrient substrates (19). Thus, we hypothesized that increasing levels of plasma albumin after surgery may prevent the occurrence of PE.
The limitations of the study are the following: (I) this was not a randomized controlled trial; and (II) we did not perform stratification according to the amount of pleural fluid. Further studies are warranted to investigate whether the PE is dependent on CRS or CRS + HIPEC.
Conclusions
In summary, patients with late-stage and recurrent ovarian cancer are prone to developing postoperative PE after CRS + HIPEC. Preoperative PE and intraoperative diaphragmatic involvement are considered independent risk factors of postoperative PE. The incidence of PE after operation can be 100%, with most cases being grade III–IV. Physicians should be aware of patients with these two risk factors and take appropriate measures for the prevention and treatment of postoperative PE.
Acknowledgments
Funding: This study was funded by the Capital characteristic application research and achievement promotion project (Z161100000516077).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2334
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2334
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2334). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Retrospective analysis of data was approved by the local ethic committee. Only data were analyzed, all participant records/information was anonymized and de-identified prior to analysis. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med 2018;378:230-40. [Crossref] [PubMed]
- Sun JH, Ji ZH, Yu Y, et al. Cytoreductive Surgery plus Hyperthermic Intraperitoneal Chemotherapy to Treat Advanced/Recurrent Epithelial Ovarian Cancer: Results from a Retrospective Study on Prospectively Established Database. Transl Oncol 2016;9:130-8. [Crossref] [PubMed]
- Li Y, Zhou YF, Liang H, et al. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies. World J Gastroenterol 2016;22:6906-16. [Crossref] [PubMed]
- Deraco M, Virzi S, Iusco DR, et al. Secondary cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent epithelial ovarian cancer: a multi-institutional study. BJOG 2012;119:800-9. [Crossref] [PubMed]
- Chua TC, Robertson G, Liauw W, et al. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol 2009;135:1637-45. [Crossref] [PubMed]
- Huo YR, Richards A, Liauw W, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: A systematic review and meta-analysis. Eur J Surg Oncol 2015;41:1578-89. [Crossref] [PubMed]
- Zhang G, Zhu Y, Liu C, et al. The prognosis impact of hyperthermic intraperitoneal chemotherapy (HIPEC) plus cytoreductive surgery (CRS) in advanced ovarian cancer: the meta-analysis. J Ovarian Res 2019;12:33. [Crossref] [PubMed]
- Cascales Campos P, Gil J, Parrilla P. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with primary and recurrent advanced ovarian cancer. Eur J Surg Oncol 2014;40:970-5. [Crossref] [PubMed]
- Uchiyama H, Harimoto N, Itoh S, et al. Pleural Effusion After Hepatectomy for Hepatocellular Carcinoma: Risk Factor Analyses and Its Impact on Oncological Outcomes. World J Surg 2017;41:1089-99. [Crossref] [PubMed]
- Ashikhmina EA, Schaff HV, Sinak LJ, et al. Pericardial effusion after cardiac surgery: risk factors, patient profiles, and contemporary management. Ann Thorac Surg 2010;89:112-8. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Di Giorgio A, De Iaco P, De Simone M, et al. Cytoreduction (Peritonectomy Procedures) Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Advanced Ovarian Cancer: Retrospective Italian Multicenter Observational Study of 511 Cases. Ann Surg Oncol 2017;24:914-22. [Crossref] [PubMed]
- Warschkow R, Tarantino I, Lange J, et al. Does hyperthermic intraoperative chemotherapy lead to improved outcomes in patients with ovarian cancer? A single center cohort study in 111 consecutive patients. Patient Saf Surg 2012;6:12. [Crossref] [PubMed]
- Mironov O, Ishill NM, Mironov S, et al. Pleural effusion detected at CT prior to primary cytoreduction for stage III or IV ovarian carcinoma: effect on survival. Radiology 2011;258:776-84. [Crossref] [PubMed]
- Miao N, Pingpank JF, Alexander HR, et al. Cytoreductive surgery and continuous hyperthermic peritoneal perfusion in patients with mesothelioma and peritoneal carcinomatosis: hemodynamic, metabolic, and anesthetic considerations. Ann Surg Oncol 2009;16:334-44. [Crossref] [PubMed]
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013;34:459-71. [Crossref] [PubMed]
- Gumaste V, Singh V, Dave P. Significance of pleural effusion in patients with acute pancreatitis. Am J Gastroenterol 1992;87:871-4. [PubMed]
- Fanfani F, Fagotti A, Gallotta V, et al. Upper abdominal surgery in advanced and recurrent ovarian cancer: role of diaphragmatic surgery. Gynecol Oncol 2010;116:497-501. [Crossref] [PubMed]
- Thévenot T, Bureau C, Oberti F, et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol 2015;62:822-30. [Crossref] [PubMed]
(English Language Editor: J. Jones)


