
Chemotherapy and targeted therapy near the end of life affects aggressiveness of palliative care
Introduction
In the past 20 years, cancer treatment has developed rapidly, with hugely improved efficacy (1). However, for patients with advanced cancer, there is also growing concerned over cancer care’s aggressiveness near the end of life (EOL) (2,3). The use of chemotherapy increases significantly in patients with advanced cancer in the last weeks of life (4-6).
When to cease chemotherapy, treatment is significantly important for the patient’s quality of life (QOL), and it is considered to be a quality assurance factor for EOL patients with cancer. Earle et al. suggested that indicators of quality of EOL palliative care should be identified, and they concluded that the continued use of chemotherapy for patients with cancer who were very near to death represented overly aggressive care (7). A Canadian study showed that factors such as cancer type, palliative care assessment, female sex, physician house calls, and utilization of home care were significantly associated with decreased use of EOL chemotherapy (4). Furthermore, aggressive care may be associated with living in an area with higher numbers of teaching hospitals and being cared for in teaching hospitals (2).
Over the past 10 years, the treatment of advanced cancer has changed dramatically. The use of palliative chemotherapy (PCT) and the range of available drugs are constantly increasing, including targeted therapy and immunotherapies (8,9). Increasing knowledge about the molecular events driving tumorigenesis will lead to more people with advanced cancer receiving targeted therapy. In breast cancer, targeted therapy such as Trastuzumab, an anti-Her2 antibody, was approved as first-line therapy in combination with chemotherapy for Her2-positive disease. Sometimes targeted therapy is more preferred to chemotherapy, patients with EGRF mutations in non-small cell lung cancer can use Iressa or Tarceva which have better overall survival than choosing chemotherapy.
In the present study, we aimed to explore the percentage of patients with metastatic cancer treated by PCT and examined treatment intensity indicators and the differences between administration of targeted therapy and chemotherapy during the last month of life. We aim to use this research to explore the current status of chemotherapy and targeted therapy for patients with advanced cancer in the last month, to avoid futile treatment, and improve palliative care for patients with terminal cancer. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1845).
Methods
Subjects
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional research ethics committee of Fudan University Shanghai Cancer Center (No. 081564-3-1051: the registration number of ethics board) and individual consent for this retrospective analysis was waived. A total of 585 patients who had received targeted therapy or PCT between April 2007 and December 2018 at the Department of Integrated Therapy of Fudan University Shanghai Cancer Center were included in this retrospective study. Exclusion criteria were: (I) patients not found by the medical oncology service; and (II) patients diagnosed with a hematological malignancy.
Data collection
The inclusion criteria comprised: patients whose information about their cancer diagnosis was deposited in the computerized medical record system at our hospital; who had received at least one cancer therapy; and had received PCT or targeted therapy as the final cancer therapy; and if the malignancy was nonhematological.
The following data were collected: (I) sociodemographic information, including age, sex, family caregivers, and cancer type; (II) clinical information, including performance status (PS), comorbidity, prior treatment, cardiopulmonary resuscitation (CPR) in the last month of life, the number of hospital admission in the last month of life, the number of intensive care unit (ICU) admissions in the last month of life, and endotracheal tube intubation in the last month of life.
Statistical analysis
Excel for Windows version 13 (Microsoft Corp., Redmond, WA, USA) and SPSS version 20.0 (IBM Corp., Armonk, NY, USA) was used to analyze the data. P<0.05 was considered to be statistically significant. Logistic regression modeling was used, the results of which are expressed as odds ratios (ORs) and their corresponding 95% confidence intervals (CIs).
Results
General clinical information
During the study period, 585 patients died of advanced cancer, and of them, 87 (14.9%) were treated with PCT, and 125 (21.3%) received targeted therapy during the last month of life (Tables 1,2). Patients aged less than 50 years old most often underwent EOL chemotherapy (P<0.001), and patients whose PS was less than 2 were likely to receive targeted therapy and chemotherapy (P<0.001). Among patients who underwent chemotherapy, the most frequent diagnoses were lung cancer (17.6%), breast cancer (16.2%), colorectal cancer (16.1%), gynecological cancer (12.1%), gastric cancer (11.4%), pancreatic cancer (10.5%), and other cancers (18.4%). The use of PCT in the last month of life was independently associated with age <50 years (OR, 1.78; 95% CI, 1.28–2.09; P<0.001) and PS <2 (OR, 2.66; 95% CI, 2.14–3.79; P<0.001). The use of targeted therapy was independently associated with PS <2 (OR, 1.52; 95% CI, 1.15–2.33; P<0.001) (Table 3).
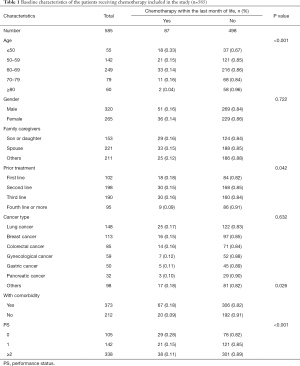
Full table
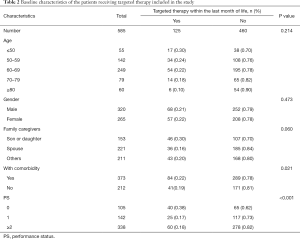
Full table
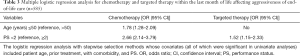
Full table
Indicators of aggressive care with targeted therapy and PCT
As for indicators of aggressive care among patients who continued to receive PCT within the last month of life, endotracheal tube intubation near death, emergency department (ED) visits during the last month of life, ICU admission, and CPR were all significantly higher (Table 4). The risk of ICU admission was significantly higher for patients who continued to receive PCT within the last month of life (OR, 2.33; 95% CI, 1.91–2.92; P<0.001), and these patients were more likely to be treated with CPR (OR, 4.18; 95% CI, 2.91–5.40; P<0.001) (Table 5).
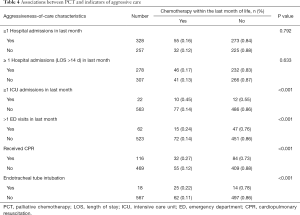
Full table

Full table
Difference between targeted therapy and PCT
We found that most lung cancer patients received targeted therapy in the terminal stage (P<0.001). We also found that receiving targeted therapy correlated with higher rates of admission to the ICU (P<0.001) (Table 6). Logistic regression-based multivariate analysis showed that lung cancer was an independent predictor of targeted therapy administration (OR, 4.18; 95% CI, 2.29–5.48; P<0.001), and admission to the ICU was an independent predictor of PCT administration (OR, 2.18; 95% CI, 1.81–2.75) (Table 7).
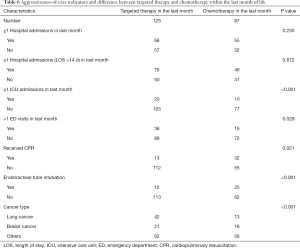
Full table

Full table
Discussion
The present study investigated the use of EOL chemotherapy and targeted therapy using data from 585 consecutive patients who died of advanced cancer. Our results showed that receiving targeted therapy correlated with lower rates of admission to the ICU in comparison with PCT.
At the EOL, more detailed conversations among physicians, caregivers, and patients could reduce aggressive treatment incidence, resulting in a significantly better QOL in the last month of life (10). However, for patients who are dying from advanced solid tumors, decision-making in EOL treatment is complex. Deciding on a more aggressive intervention involves access to a hospice, knowledge, and attitude of the patients and their families toward hospices and PCT, and their relationship with primary and specialist physicians. Unfortunately, the administrative data used in this study did not include these preferences.
Some physicians from cancer-specialty hospitals have systematically studied palliative care guidelines, but as a rule, physicians overestimate prognosis by at least 30% (11). Those from general hospitals lack professional palliative care knowledge, and it is difficult for many oncologists and caregivers to provide the option of stopping chemotherapy (12,13). Hesitation regarding cessation of futile PCT might lead to more aggressive EOL care and life-sustaining treatment. In China, some caregivers ask physicians not to tell the truth concerning the patient’s condition, so that patients fail to receive the full picture regarding their disease and do not understand the purpose of palliative care, and they believe that their diseases are curable (14). Patients thus have high expectations of palliative therapy and will accept the adverse events and toxicity to prolong their survival (15). It has been reported that patients would prefer to receive aggressive chemotherapy, even if it only prolonged their life for 1 week because most patients do not receive enough information about the benefits of PCT (16).
Our study showed that in the last month of life, 14.9% of patients received PCT, which was in line with international recommendations. However, a Portuguese study indicated that the prevalence of chemotherapy within the last month of life could be as high as 37% (17). One reason is that in more affluent countries, patients have a high chance of receiving a medicalized death and futile treatments (18). Another explanation is that older EOL patients in China are more inclined to receive traditional Chinese medicine (TCM) than Western medicine, which would lead to the low use of chemotherapy.
Our results showed that younger patients (<50 years old) with a PS <2 received more aggressive EOL chemotherapy and targeted therapy. There are several possible explanations for this. Patients younger than 50 years old mostly had better education and tended to choose Western medicine in preference to TCM, explaining why this subgroup was more likely to receive PCT. The younger patients also had lower rates of comorbidity and received more rounds of cytotoxic chemotherapy. There is a complex relationship between aggressive care and comorbidity. Patients with comorbid conditions were less likely to receive chemotherapy. If they did receive it, they were more likely to be admitted to the ICU or go to the hospital in the final month of life (4). Admission to ICU and receipt of CPR were more frequent in patients treated with PCT than in patients who received targeted therapy in the last month of life. Previously, the therapeutic options of patients with advanced cancer were limited to cytotoxic chemotherapy. Over the past decade, cancer treatment, such as advanced non-small cell lung cancer, has changed markedly. Successful therapies that target patients with anaplastic lymphoma kinase rearrangements or epidermal growth factor receptor mutations have been developed (19-21). In cases of a specific oncogenic driver gene being identified, most people will choose targeted therapy. Therefore, targeted therapy was used more often than traditional chemotherapy agents that often have lower toxicity and fewer complications. Our results showed that patients who received targeted therapy received less aggressive treatment, such as ICU admission and CPR.
Every individual at the EOL has a right to pursue life prolongation at any cost. We cannot consider that their aggressive care is unnecessary. However, mounting evidence suggests that most dying patients do not desire such care (22-24). Research also suggests that less aggressive care is cheaper and less upsetting for surviving members of the patient’s family (10,25-27). Accordingly, the arguments in favor of less aggressive EOL care suggest that discussions about changing EOL care should occur earlier to ensure that palliative treatment is consistent with the patient’s preferences.
According to these data, we hypothesize that the adverse events or toxicity associated with intravenous chemotherapy outweigh its benefit over the whole treatment course, especially at EOL. Considering the increasing availability of targeted therapies, we require a deeper understanding of EOL care roles. Also, in this study, we lacked detailed knowledge about the factors affecting EOL chemotherapy decision making among physicians, patients, and their caregivers, such as the values and beliefs of the patients and physicians and discussion about decision making on EOL care. These factors will also contribute to patients’ choices at EOL, for example, ED visits, admission to ICU, increased hospital admissions, or decreased hospice use (28).
There are also some limitations to our study. Firstly, one significant limitation of this study is a single-center study only, and it was a retrospective study that relied on hospital records. Secondly, we did not analyze the role of targeted therapy in the terminal stage. Also, a detailed analysis of the relationship between PCT and survival rate of patients was not performed.
Conclusions
The present study explored the prevalence of targeted therapy and PCT for Chinese patients with solid cancers. During their last month of life, 14.9% of patients with advanced cancer received PCT, and 21.3% underwent targeted therapy. Most patients who received PCT preferentially received CPR and admission to the ICU compared to patients who received targeted therapy. However, the role of targeted therapy requires further study. Further palliative care guidelines are needed to enhance the quality of EOL care.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1845
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1845
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1845). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional research ethics committee of Fudan University Shanghai Cancer Center (No. 081564-3-1051: the registration number of ethics board) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dizon DS, Krilov L, Cohen E, et al. Clinical cancer advances 2016: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol 2016;34:987-1011. [Crossref] [PubMed]
- Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol 2008;26:3860-6. [Crossref] [PubMed]
- Martoni AA, Tanneberger S, Mutri V. Cancer chemotherapy near the end of life: the time has come to set guidelines for its appropriate use. Tumori 2007;93:417-22. [Crossref] [PubMed]
- Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 2004;22:315-21. [Crossref] [PubMed]
- Ho TH, Barbera L, Saskin R, et al. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol 2011;29:1587-91. [Crossref] [PubMed]
- Liu TW, Chang WC, Wang HM, et al. Use of chemotherapy at the end of life among Taiwanese cancer decedents, 2001-2006. Acta Oncol 2012;51:505-11. [Crossref] [PubMed]
- Earle CC, Park ER, Lai B, et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 2003;21:1133-38. [Crossref] [PubMed]
- Naylor EC, Desani JK, Chung PK. Targeted therapy and immunotherapy for lung cancer. Surg Oncol Clin N Am 2016;25:601-9. [Crossref] [PubMed]
- Guan LY, Lu Y. New developments in molecular targeted therapy of ovarian cancer. Discov Med 2018;26:219-29. [PubMed]
- Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med 2009;169:480-8. [Crossref] [PubMed]
- Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 2003;327:195-8. [Crossref] [PubMed]
- The AM. Collusion in doctor-patient communication about imminent death: an ethnographic study. BMJ 2000;321:1376-81. [Crossref] [PubMed]
- Cavalli-Björkman N, Glimelius B, Strang P. Equal cancer treatment regardless of education level and family support? A qualitative study of oncologists' decision-making. BMJ Open 2012;2:e001248 [Crossref] [PubMed]
- Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319-26. [Crossref] [PubMed]
- Patnaik A, Doyle C, Oza AM. Palliative therapy in advanced ovarian cancer: Balancing patient expectations, quality of life and cost. Anticancer Drugs 1998;9:869-78. [Crossref] [PubMed]
- Wright AA, Zhang B, Keating NL, et al. Associations between palliative chemotherapy and adult cancer patients’ end of life care and place of death: Prospective cohort study. BMJ 2014;348:g1219. [Crossref] [PubMed]
- Braga S, Miranda A, Fonseca R, et al. The aggressiveness of cancer care in the last three months of life: a retrospective single centre analysis. Psychooncology 2007;16:863-68. [Crossref] [PubMed]
- Goh CR. Medicalization of dying: are we turning the corner? J Palliat Med 2012;15:728-29. [Crossref] [PubMed]
- Bild AH, Yao G, Chang JT, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006;439:353-7. [Crossref] [PubMed]
- Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer 2010;10:760-74. [Crossref] [PubMed]
- Tseng HH, He B. Molecular markers as therapeutic targets in lung cancer. Chin J Cancer 2013;32:59-62. [Crossref] [PubMed]
- Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665-73. [Crossref] [PubMed]
- Mack JW, Weeks JC, Wright AA, et al. End-of-life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 2010;28:1203-8. [Crossref] [PubMed]
- Weeks JC, Cook EF, O’Day SJ, et al. Relation-ship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 1998;279:1709-14. [Crossref] [PubMed]
- Brumley R, Enguidanos S, Jamison P, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J Am Geriatr Soc 2007;55:993-1000. [Crossref] [PubMed]
- Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med 2008;11:180-90. [Crossref] [PubMed]
- Wright AA, Keating NL, Balboni TA, et al. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol 2010;28:4457-64. [Crossref] [PubMed]
- Fang P, Jagsi R, He W, et al. Rising and falling trends in the use of chemotherapy and targeted therapy near end of life in older patients with cancer. J Clin Oncol 2019;37:1721-31. [Crossref] [PubMed]


