
A retrospective review of the use of oxymorphone immediate release for long term pain control in cancer patients with gastrostomy tubes
Introduction
More than 80% of advanced cancer patients experience pain and require opioids (1-4). Up-titration of opioid doses to achieve sufficient analgesia may cause accumulation of opioid metabolites leading to the development of opioid-induced neurotoxicity (OIN) (5). Opioid rotation (OR), the substitution of one opioid by another, is recommended in situations such as OIN and uncontrolled pain despite opioid up-titration (6). Approximately 30–50% of cancer patients treated by palliative care teams will require an OR (6,7). OR is also performed when a change in the route of administration is required, such as in cases of oral mucositis, dysphagia, and bowel obstruction (8-16).
The placement of a percutaneous endoscopic gastrostomy-tube (G-tube) in patients with dysphagia allows for the administration of opioids per-tube. Only methadone and immediate-release (IR) preparations of opioids can be administered per-tube, except for newer extended-release (ER) preparations of morphine and oxycodone which can be opened and the contents administered via G-tubes (8-10). However, these preparations are expensive and contraindicated in patients with bowel obstruction, ileus, and venting G-tubes (8-11). Venting G-tubes are frequently placed in patients with bowel obstruction and allows for orally administered IR opioids to be absorbed higher up in the gastrointestinal (GI) tract when the G-tube is temporarily clamped after administration. In patients with dysphagia or bowel obstruction, long-acting formulations of opioids are restricted to fentanyl patch due to its transdermal delivery, and methadone due to its pharmacokinetic properties (11-16), while conventional ER formulations of morphine, oxycodone, and hydromorphone are contraindicated (11). Transmucosal rapid-acting fentanyl products are useful only for breakthrough pain (12).
Oxymorphone is a potent semi-synthetic mu-opioid agonist initially approved in the 1950s. It is available in both IR and ER formulations.
In contrast to other IR oral opioids, oxymorphone IR has a longer half-life and takes a shorter time to attain peak concentration allowing for dosing every 8 hours. Oxymorphone does not utilize the cytochrome P-450 system and has minimal drug interactions. Adverse effects observed are no different than any other opioids, such as morphine, oxycodone, and hydromorphone (17-20). Oxymorphone IR tablet’s unique features of a long half-life, rapid absorption from the upper GI tract, and ability to be crushed and administered via G-tubes, makes for an attractive alternative to fentanyl and methadone for long-acting pain control in patients with G-tubes. Our goal was to determine the proportion of successful OR to oxymorphone in cancer patients with G-tubes and to determine the OR ratio (ORR) from morphine equivalent daily dose (MEDD) to oxymorphone. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-969).
Methods
This retrospective study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center (PA17-0385) and conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. We reviewed the charts of cancer patients with G-tubes between the years 2014 and 2017 to identify patients that underwent OR to oxymorphone IR. All cases from this time window were included in the data analysis to maximize the sample size and avoid selection bias. Data regarding patient characteristics, opioid use, MEDD, and scores on the Edmonton Symptom Assessment Scale (ESAS) (21), Memorial Delirium Assessment Scale (MDAS) (22), and Cut-down, Annoyed, Guilty, Eye-opener (CAGE) (23) questionnaire were obtained. Criteria previously reported by our team defining successful OR were used in our study (6,24-27). These criteria include: (I) a 30% or 2-point reduction in the ESAS pain [0–10] score, for OR due to uncontrolled pain; or (II) evidence of the disappearance of side effects at the follow-up visit for OR due to OIN; or (III) no worsening of pain score for OR performed due to change in route of administration or drug interaction; and (IV) continued use of oxymorphone at the time of follow-up in outpatients and discharge in inpatients.
ORR was calculated as the baseline pre-rotation MEDD mg/oxymorphone mg/day at the time of follow-up (outpatients) or discharge (inpatients) in patients who underwent a successful OR (6,24-27). The MEDD calculated based on the MEDD table used by our team (28).
Statistical analysis
Data were summarized using standard descriptive statistics such as mean, standard deviation, median, interquartile range (IQR), and range for continuous variables; frequency and proportion for categorical variables. Association between categorical variables was examined by the Chi-Squared test or Fisher’s exact test when appropriate. Wilcoxon rank-sum test or Kruskal-Wallis test was used to examine the difference in continuous variables. Univariate logistic regression models were applied to assess the effect of variables of interest on the success of OR. All computations were carried out in SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Forty patients with G-tubes underwent OR from other strong opioids to oxymorphone. The median age was 56 years, 27 (67.5%) were male, 23 (57.5%) were white, and 36 (90%) had advanced-stage cancer (Table 1). Twenty-seven (67.5%) were inpatients and 13 (32.5%) outpatients. Only 6/40 patients had venting G-tubes and received the medications orally with clamping of the G-tube, and the remainder had feeding tubes and received medications per-tube. Uncontrolled pain was the most common (95%) reason for OR.
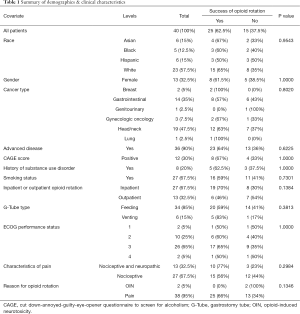
Full table
Of the 40 patients, 27 continued to use oxymorphone at the time of discharge from the inpatient setting or at the time of follow-up in the outpatient setting, and 25 (62.5%) met the criteria for successful OR. Among the 15 patients with unsuccessful OR, five did not return for a follow-up, seven had insufficient improvement in pain requiring OR to another opioid, and three passed away in the palliative care unit and did not meet the criteria of successful discharge.
There were no independent predictors for successful OR. The median ORR from MEDD to oxymorphone was 3.5 (IQR, 3.1–4) in the 25 patients with successful OR (Table 2). The median ORR did not vary according to gender, cancer type, history of substance use, CAGE status, G-tube (feeding or venting), setting of OR (inpatient or outpatient), or baseline MEDD. The median ORR was 3.5 in patients with MEDD of <100 mg (11 patients) and ≥100 mg (14 patients).
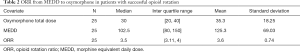
Full table
There were no reports of any unusual side effects or discontinuation of oxymorphone related to G-tube administration or clamping of the venting G-tube.
Discussion
ORs in cancer patients have a success rate of ≥50% (29). ORs from MEDD to oxymorphone IR in cancer patients with G-tubes had a success rate of 62.5% in our study. Due to its long half-life, oxymorphone IR was successfully used in cancer patients with feeding tubes and conferred long term analgesia when administered every 8 hours around the clock. In contrast, other IR opioids have a half-life of ≤4 hours and require administration every 4 hours for efficient pain management. The need for six doses of IR opioids timed 4 hours apart may subject patients to uncontrolled pain associated with missed or delayed doses, and are cumbersome to administer at night interfering with sleep.
Oxymorphone is more lipophilic than morphine, hydromorphone, or oxycodone, resulting in quick absorption through the GI tract, faster penetration of the blood-brain barrier, and attainment of peak concentration in only 30 minutes. Due to its rapid absorption and long half-life, oxymorphone IR can be successfully used orally in patients with venting G-tubes for bowel obstruction. In these patients, oral administration of oxymorphone IR should be followed by clamping of the G-tube for 30 minutes.
The recommended ORR from oral morphine to oxymorphone is 3 (30 mg of morphine =10 mg of oxymorphone) (30). In our study, the median ORR from MEDD to oxymorphone in 25 cancer patients with G-tubes who underwent a successful OR was 3.5. Due to our small sample size, our group recommends that an ORR of 3 continue to be used for OR from MEDD to oxymorphone in patients with G-tubes. As always, close patient monitoring after OR is required. Unlike our previous OR studies involving hydrocodone, hydromorphone, and transdermal fentanyl, the ORR in this study did not differ according to the pre-rotation opioid dose (24-27).
Oxymorphone may have specific benefits when compared to other opioids. It has less potential for drug interactions since it does not involve the cytochrome P450 enzymes. Oxymorphone is metabolized by uridine diphosphate glucuronosyltransferase (UGT) to form oxymorphone 3-glucuronide (inactive metabolite), and 6-hydroxy-oxymorphone which can accumulate in renal failure (31). Oxymorphone must be prescribed with caution in patients with renal insufficiency and avoided in renal failure, similar to morphine, oxycodone, and hydromorphone. Oxymorphone is not highly protein bound and does not promote histamine release and hence may be helpful in patients with hypoalbuminemia and opioid-related pruritis. It is recommended that oxymorphone be taken on an empty stomach—at least 1 hour before or 2 hours after a meal. However, in clinical studies, oxymorphone did not demonstrate any clinically meaningful difference in absorption when taken with meals (18). Caution must be exercised with avoidance of oxymorphone administration closer to the timing of G-tube feeds.
Our study has some limitations, including its retrospective design, small sample size, and enrollment of both inpatient and outpatient populations. Well-designed prospective studies in defined outpatient or inpatient populations must be conducted in the future to investigate the use of oxymorphone in patients with G-tubes, and to determine the ORR for OR from MEDD to oxymorphone.
In summary, oxymorphone IR offers another alternative to methadone, transdermal fentanyl, and newer ER opioid capsule preparations for long term pain control in cancer patients with feeding tubes and is an attractive option in patients with venting G-tubes where all ER oral opioid preparations are contraindicated.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-969
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-969
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-969). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center (PA17-0385) and conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- . Cancer pain relief and palliative care. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1990;804:1-75. [PubMed]
- van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, et al. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J Pain Symptom Manage 2016;51:1070-90.e9. [Crossref] [PubMed]
- Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 1994;330:592-6. [Crossref] [PubMed]
- Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012;13:e58-68. [Crossref] [PubMed]
- Lötsch J. Opioid metabolites. J Pain Symptom Manage 2005;29:S10-24. [Crossref] [PubMed]
- Reddy A, Yennurajalingam S, Pulivarthi K, et al. Frequency, outcome, and predictors of success within 6 weeks of an opioid rotation among outpatients with cancer receiving strong opioids. Oncologist 2013;18:212-20. [Crossref] [PubMed]
- Mercadante S, Ferrera P, Villari P, et al. Frequency, indications, outcomes, and predictive factors of opioid switching in an acute palliative care unit. J Pain Symptom Manage 2009;37:632-41. [Crossref] [PubMed]
- McCarberg BH, Kopecky EA, O'Connor M, et al. An abuse-deterrent, microsphere-in-capsule formulation of extended-release oxycodone: alternative modes of administration to facilitate pain management in patients with dysphagia. Curr Med Res Opin 2016;32:1975-82. [Crossref] [PubMed]
- Fleming AB, Carlson DR, Varanasi RK, et al. Evaluation of an Extended-Release, Abuse-Deterrent, Microsphere-in-Capsule Analgesic for the Management of Patients with Chronic Pain With Dysphagia (CPD). Pain Pract 2016;16:334-44. [Crossref] [PubMed]
- Nicholson B. Morphine sulfate extended-release capsules for the treatment of chronic, moderate-to-severe pain. Expert Opin Pharmacother 2008;9:1585-94. [Crossref] [PubMed]
- Didwaniya N, Reddy A, Gottumukkala RS, et al. What is your gut feeling about opioid rotation? J Clin Oncol 2015;33:e11-2. [Crossref] [PubMed]
- Swarm RA, Abernethy AP, Anghelescu DL, et al. Adult cancer pain. J Natl Compr Canc Netw 2013;11:992-1022. [Crossref] [PubMed]
- Ripamonti CI. Malignant bowel obstruction: tailoring treatment to individual patients. J Support Oncol 2008;6:114-5. [PubMed]
- Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer 2008;44:1105-15. [Crossref] [PubMed]
- Dolan EA. Malignant bowel obstruction: a review of current treatment strategies. Am J Hosp Palliat Care 2011;28:576-82. [Crossref] [PubMed]
- Tuca A, Guell E, Martinez-Losada E, et al. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res 2012;4:159-69. [Crossref] [PubMed]
- Prommer E. Oxymorphone: a review. Support Care Cancer 2006;14:109-15. [Crossref] [PubMed]
- Sloan PA, Barkin RL. Oxymorphone and oxymorphone extended release: a pharmacotherapeutic review. J Opioid Manag 2008;4:131-44. [Crossref] [PubMed]
- Vadivelu N, Maria M, Jolly S, et al. Clinical applications of oxymorphone. J Opioid Manag 2013;9:439-52. [Crossref] [PubMed]
- Chamberlin KW, Cottle M, Neville R, et al. Oral oxymorphone for pain management. Ann Pharmacother 2007;41:1144-52. [Crossref] [PubMed]
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6-9. [Crossref] [PubMed]
- Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage 1997;13:128-37. [Crossref] [PubMed]
- Bush B, Shaw S, Cleary P, et al. Screening for alcohol abuse using the CAGE questionnaire. Am J Med 1987;82:231-5. [Crossref] [PubMed]
- Reddy A, Tayjasanant S, Haider A, et al. The opioid rotation ratio of strong opioids to transdermal fentanyl in cancer patients. Cancer 2016;122:149-56. [Crossref] [PubMed]
- Reddy A, Vidal M, Stephen S, et al. The Conversion Ratio From Intravenous Hydromorphone to Oral Opioids in Cancer Patients. J Pain Symptom Manage 2017;54:280-8. [Crossref] [PubMed]
- Reddy A, Yennurajalingam S, Desai H, et al. The opioid rotation ratio of hydrocodone to strong opioids in cancer patients. Oncologist 2014;19:1186-93. [Crossref] [PubMed]
- Reddy A, Yennurajalingam S, Reddy S, et al. The Opioid Rotation Ratio From Transdermal Fentanyl to "Strong" Opioids in Patients With Cancer Pain. J Pain Symptom Manage 2016;51:1040-5. [Crossref] [PubMed]
- Dalal S, Bruera E, editors. The M.D. Anderson supportive and palliative care handbook. Fifth ed. Houston, TX: The University of Texas Health Science Center at Houston, 2015.
- Mercadante S, Bruera E. Opioid switching: a systematic and critical review. Cancer Treat Rev 2006;32:304-15. [Crossref] [PubMed]
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recommendations and reports MMWR Recomm Rep 2016;65:1-49. [Crossref] [PubMed]
- Smith HS. Clinical Pharmacology of Oxymorphone. Pain Med 2009;10:S3-S10. [Crossref]


