
A randomized trial of the dural puncture epidural technique combined with programmed intermittent epidural boluses for labor analgesia
Introduction
In recent years, epidural labor analgesia has commonly been provided via the patient-controlled epidural analgesia (PCEA) with continuous epidural infusion (CEI) technique (1,2). Adding PCEA to CEI has resulted in decreased use of local anesthetics, a lower motor block, and greater patient satisfaction, which can minimize clinicians’ need for emergency injection intervention (3,4). However, even though PCEA is used in conjunction with CEI, some patients may experience breakthrough pain, requiring an anesthesiologist's attention. The rate of incidence may depend on the understanding and proper use of PCEA by the patient, as well as on the PCEA/CEI settings. The programmed intermittent epidural bolus (PIEB) is an automated method for administering epidural local anesthetic solutions (with or without opioids) at fixed, scheduled intervals. It is used only as an alternative to CEI or as part of a background infusion of local anesthesia in PCEA labor analgesia techniques. Previous randomized controlled trials (5,6) have shown that using a PIEB instead of background infusion can reduce the consumption of local anesthetics as well as the incidence of maternal motor block and breakthrough pain, thus making the need for anesthesiologist rescue supplements less urgent. Recently, the dural puncture epidural (DPE) technique has attracted increasing research attention and popularity due to its improved blocking and fewer side effects in the obstetric population (7,8). DPE means that the operator puncture the dural with a spinal needle through the epidural needle without adding any drugs. Its unique advantages may be more appropriate for labor analgesia, though this remains controversial, and whether it is beneficial for initiating labor analgesia and the quality of analgesia has not been determined (9). CEI means Continuous epidural infusion (CEI) means continuous infusion into epidural space. The PIEB technique is commonly used in labor analgesia, however few applicable case-control studies on its combination with DPE are available. We speculate that the concomitant use of the DPE technique for initiating labor analgesia and the PIEB mode of maintenance may provide greater benefits than the CEI technique combined with PIEB in accelerating the onset of analgesia, improving the sacral block, reducing the local anesthetic dosage, lowering the incidence of side effects, and enhancing maternal satisfaction. Therefore, we conducted a prospective randomized controlled study to compare and evaluate the efficacy and safety of DPE combined with PIEB and CEI combined with PIEB for labor analgesia.
We present the following article in accordance with the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2281).
Methods
Research and design
This is a prospective randomized cohort trial approved by the institutional ethics committee of Fujian Maternal and Child Health Hospital (Ethics No. 168 June 12, 2017) and adhered to the Declaration of Helsinki (as revised in 2013). The study was registered at Chictr.org.cn (No. ChiCTR1900020852; date of registration: January 21, 2019) before patient enrollment, and conforms to the applicable EQUATOR (Enhancing the Quality and Transparency of Health Research) guidelines.
Participants
Written informed consent was obtained from all participants. Women were eligible for inclusion in this study based on the following criteria: (I) healthy nulliparous; (II) full-term (37–42 weeks); (III) American Society of Anesthesiologists class I–II; (IV) height 150–170 cm; (V) body weight 60–100 kg; (VI) those with spontaneous labor pain with a visual analogue scale (VAS) score >50 mm (VAS score: 0=no pain, 100=worst pain imaginable); (VII) cervical dilatation between 2 and 5 cm; and (VIII) those who required neuraxial analgesia. The exclusion criteria were as follows: (I) those that refused to participate; (II) cases complicated with cardiac and brain dysfunction; (III) those who received preoperative injection of opioids; (IV) those with spinal deformity; (V) women with a multiple pregnancy; (VI) preterm delivery; (VII) those with diabetes or gestational diabetes; (VIII) those with contraindications for epidural block; and (IX) women who underwent cesarean section before complete dilatation of the cervix after analgesia, or delivery within 1 hour after epidural catheterization.
Randomization and concealment of the groups
Assignments
Women enrolled in this study were randomized to two groups through a computer-generated random number sequence (CEI + PIEB, DPE + PIEB; n=100 in each group). To maintain the blindness of the study, two anesthesia providers were involved when the participants requested neuraxial analgesia; one anesthesia provider attended the operating room to perform the neuraxial analgesia procedure, while the other remained outside the operating room. Data collection was not approved until a neuraxial analgesia procedure was completed so that neither the blinded assessor nor the participants could see whether CEI + PIEB or DPE + PIEB was employed to administer the initial epidural medications.
Initiation of labor analgesia
Baseline heart rate, non-invasive arterial blood pressure (average of three readings during uterine contraction intervals), and blood oxygen saturation were recorded after admission, and 500 mL of lactate Ringer’s solution was infused.
Epidural puncture operation method: the L3–L4 space was determined under ultrasound guidance, a 17-G epidural needle was used, and standard loss of resistance to saline technology was used to determine entry into the epidural space. In the CEI + PIEB group, after confirming the epidural space, a 19-gage epidural catheter (HeNan TuoRen, China) impregnated with a stainless steel multiorifice was inserted 4 cm into the epidural space. In the DPE + PIEB group, a 25-gauge Whitacre needle (HeNan TuoRen, China) with cerebrospinal fluid (CSF) confirmation was used for dural puncture. Next, the epidural catheter was placed 4 cm into the epidural space. Three milliliters of 1.5% lidocaine (Shanghai Harvest, China) with 15 µg of epinephrine (Shanghai Harvest, China) were given as a test dose after a negative aspiration for blood and CSF. Labor analgesia was initiated with 10 mL of 0.08% ropivacaine (AstraZeneca, Sweden) with 0.4 µg/mL of sufentanil (Yichang HumanWell, China).
Maintenance of labor analgesia
Epidural infusions contained 0.08% ropivacaine with 0.4 µg/mL of sufentanil. For both groups, the epidural pump (Jiangsu Apon, China) was designed to provide an initial dose of 10 mL and background infusion at 10 mL/h. All pumps were programmed for a PCEA bolus of 5 mL with a 20-minute lockout.
Inadequate analgesia protocol
After using PCEA twice within 20 minutes, if the patient still failed to meet the requirements for breakthrough pain, 5 mL boluses of 0.2% ropivacaine were given, with a further 5 mL given again after a 10-minute interval. If a total of 10 mL still did not provide an analgesic effect, the catheter was considered to have failed to meet the analgesic requirements, and the woman was removed from the study.
Primary outcome assessment
The patients’ baseline VAS scores were recorded when labor analgesia was required, and marked as “time zero”. Parturients were then advised to observe their “level of pain” every 2 minutes (up to 20 minutes) on a VAS 100-mm. The VAS scale was used to assess the time at which adequate pain control (VAS ≤30 mm) was achieved. The primary outcome was the percentage of participants in the two groups with adequate analgesia 10 minutes after the initiation of the epidural bolus. Adequate analgesia was defined as a VAS score of ≤30 mm for two consecutive contractions.
Secondary outcome assessments
The analgesic effect was evaluated as follows: within 30 minutes after the first administration, VAS scores were recorded at 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, and 30 minutes. After the first 30 minutes, evaluations continued at 90-minute intervals until 5 hours or delivery. The following parameters were also assessed: the number of cases with blocks reaching S2 within 20 minutes, sensory blockade level to the ice, the modified Bromage score, the times of PCEA boluses, and the need for additional boluses by the provider at any point in the labor. The total (epidural pump + PCEA + provider bolus) and hourly (total consumption divided by the duration of labor analgesia mg/h) consumption of ropivacaine were calculated. Upon delivery of the fetus, all parturients stopped epidural analgesia for 2 hours.
Other collected data
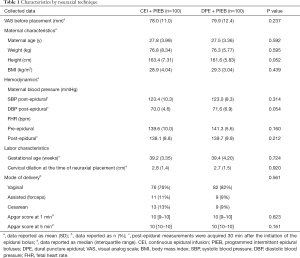
We also collected specific data including demographic statistics, obstetric variables, procedural variables, frequency of side effects, and patient satisfaction. The demographic data included maternal age, weight, height, and body mass index (BMI). Obstetrical data included maternal delivery mode, gestational age, and cervical dilation at the time of epidural placement. Procedural variables included maternal blood pressure (pre- and post-epidural), fetal heart rate (pre- and post-epidura), Apgar score, and time to pain relief.
The side effects examined included maternal hypotension, nausea, vomiting, pruritus, fetal bradycardia, and headache characteristic of 24 hours post-dural puncture. Patient satisfaction was measured on a 100-mm VAS (left “very dissatisfied” and right “very satisfied”) at 24–48 hours postpartum. Maternal hypotension was defined as a systolic blood pressure of <90 mmHg or a decrease of >20% from baseline. If hypotension occurred, rapid intravenous fluid infusion and positioning into a left or right supine position were performed simultaneously. Phenylephrine (40–80 µg) was administered intravenously if there was no improvement. Fetal bradycardia was defined as a heart rate of <110 bpm, and duration of >10 minutes. Pruritus and nausea were assessed on the following scale: 0, none; 1, mild; 2, moderate; and 3, severe.
Statistical analysis
Statistical analysis was performed using SPSS software (26.0 Version, IBM Corp, Armonk, NY, USA). Descriptive statistics were calculated for all variables in the data set of all patients. The median time to achieving pain control via the neuraxial technique was estimated using a Kaplan-Meier approach, and a 95% confidence interval (CI) around the median was reported. Univariate Cox regression models were used to assess the relative risk of achieving appropriate pain control through neuraxial techniques. Normally distributed output data are presented as the mean [standard deviation (SD)] and were compared using the t-tests. Skewed data are summarized as the median (interquartile range). The Mann-Whitney U test was used to compare the variables ordered between groups. Categorical variables are reported as frequencies (percentages) and were compared using the chi-squared (χ2) test. Statistical significance at a P value of <0.05 was assumed.
Sample size
Based on an earlier study by Wilson et al. (7), which demonstrated that the percentage of participants with adequate analgesia at 10 minutes did not differ between the neuraxial techniques, we estimated that 40% and 60% of subjects in the CEI + PIEB and DPE + PIEB groups, respectively, would achieve adequate analgesia at 10 minutes. An a priori power analysis found that a sample size of 95 per group would provide 80% power at significance level α=0.05. Due to the expected 10–20% drop in the number of patients, the sample size was increased to 105 participants per group (210 in total).
Results
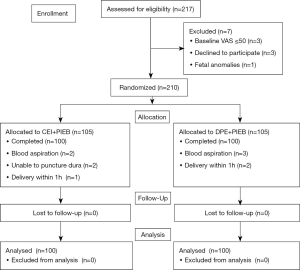
From February 2019 to June 2020, 217 women were screened, and 210 subjects were recruited. Ten participants were excluded after randomization (Figure 1), and thus, data were collected from 200 subjects. Patient characteristics appeared to be relatively balanced between the neuraxial techniques (Table 1).
Full table
Primary outcome
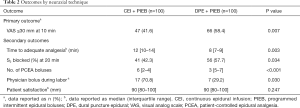
The primary outcome was that the percentage of women with adequate labor analgesia after 10 minutes of an epidural catheter bolus in the DPE + PIEB group was higher compared to the CEI + PIEB group (Table 2). Most participants (56.5%) reported adequate analgesia within 10 minutes.
Full table
Secondary outcomes
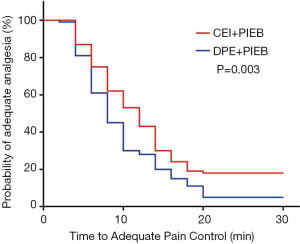
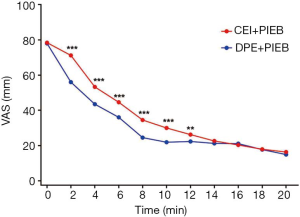
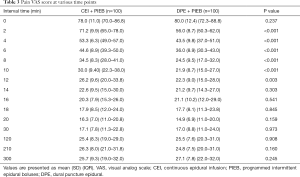
Figure 2 displays the Kaplan-Meier curves for the time to achieve adequate neuraxial analgesia. The median time to adequate analgesia was shorter in the DPE + PIEB group (Table 2). The proportion of patients in the DPE + PIEB group with VAS pain scores of ≤30 mm was significantly higher compared to the CEI + PIEB group [hazard ratio (HR) =1.488; 95% CI, 1.105–2.002; P=0.002]. The median time (95% CI) until adequate analgesia was achieved was 8 minutes (7–9 minutes) in the DPE+PIEB group and 12 minutes (10–14 minutes) in the CEI+PIEB group. Figure 3 displays the median VAS scores found at each data collection point in all patients. The VAS score decreased with time (P <0.001). When evaluating the relationship between neuraxial technique and time, VAS scores decreased more rapidly with DPE + PIEB versus CEI + PIEB (P=0.003; Figure 3). Table 3 shows the pain VAS pain scores of the two groups.
Full table
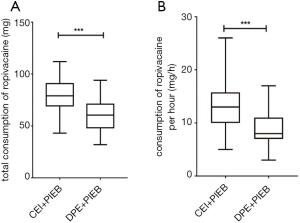
S2 sensory blocks were more frequently observed in DPE + PIEB patients at 20 minutes (P=0.034). In the DPE + PIEB group, the numbers of PCEA boluses and provider boluses were significantly lower (Table 2). Similarly, the total and hourly consumption of ropivacaine was also lower in the DPE + PIEB group (Figure 4).
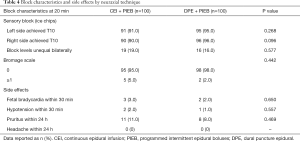
There was no significant difference in patient satisfaction during the course of labor between the neuraxial techniques (Table 2). Also, block characteristics and side effects did not vary between the neuraxial techniques (Table 4).
Full table
In the 20-minute study period, 15 cases of CEI + PIEB (15%) and seven cases of DPE + PIEB (7%) patients did not achieve consistent VAS scores of ≤30 mm. Despite this, all of the patients were satisfied with their neuraxial analgesia, and no epidural catheters were replaced.
Discussion
In our study, the percentage of parturients with adequate analgesia within 10 minutes of epidural bolus initiation from DPE + PIEB was higher than that from CEI + PIEB. This was related to three factors: (I) shorter time to adequate analgesia; (II) less local anesthetic drugs; and (III) better sacral block.
The neuraxial analgesia for labor implemented in our study was epidural analgesia, in which low concentration of ropivacaine is infused into the epidural space. Epidural analgesia can produce a selective sensory block from T10 to L1 and retain lower limb motor function.
With DPE, a spinal needle was inserted into the user's epidural needle to puncture the dura. However, no local anesthetic was injected into the subarachnoid area. Previous studies (7,10,11) found that DPE had a faster analgesia onset time than CEI, enhanced the quality of the block, improved sacral coverage, resulted in a less asymmetric block, and required less physician bolus intervention (8). However, no difference was found in the analgesia onset time between DPE and CEI in other studies (12).
PIEB is a new delivery technique. Due to the high injection pressure, the drug can be rapidly administered into the epidural cavity through the anterior and lateral segments of the epidural catheter, with a more uniform distribution (13). PIEB can provide the same (or even better) analgesic effect with fewer drug doses (14), reduce maternal motor block, and minimize the need for vacuum extraction and forceps delivery (15). Additionally, the second stage of labor for the primiparas was significantly shortened, and the total dosage of analgesics was reduced (6).Our results indicate that DPE + PIEB is an effective technique for epidural administration and may have some clinical advantages over CEI + PIEB for labor analgesia, which is consistent with previous studies.
Our data indicated that DPE + PIEB was associated with a reduced median time to adequate analgesia. We found that the VAS score of the DPE + PIEB group was lower than that of the CEI + PIEB group within 12 minutes of analgesia, and there was no difference between the two groups after 12 minutes. Contrary to our observations, Cappiello et al. (11) observed that the VAS score of the DPE group had decreased by 20 minutes, but that before the onset of analgesia or at any time interval after 20 minutes, there was no difference in the VAS score. This discrepancy could be due to the use of ropivacaine in our study, while Cappiello et al. (11) used bupivacaine. Different local anesthetic drugs can result in dissimilar observations, especially considering that the drug’s ability to spread is a factor that affects the passage of the drug through the dural pore (16). Another possible reason is that different races and analgesic techniques may also lead to varying observations.
We also observed that in DPE patients, S2 sensory blocks were more frequent at 20 minutes, which is in line with most studies evaluating sacral blocks with the DPE technique. However, the effect of the sacral block was also controversial (7,8,11,12). The conflicting results could be explained by the smaller size of the needle and the local anesthetics (12).
In this study, the times of PCA (patient-controlled analgesia) compression, as well as the total and hourly ropivacaine consumption in the DPE + PIEB group were markedly lower compared to the CEI + PIEB group. Possible explanations for this are as follows: firstly, in our study, DPE was used in combination with PIEB technology to increase the bolus pressure. Local anesthetics follow the pressure gradient and are easier to "press" from the epidural space into the subarachnoid space through the puncture hole, making the drug spread more widely (16). Secondly, the volume of local anesthetics and opioids between the epidural space to the subarachnoid space depends on the size of the spinal needle (11,17). Thirdly, local anesthetics have varying degrees of diffusion. For example, the transmembrane flow of bupivacaine is slower than that of lidocaine (18). In this study, ropivacaine was used, and further research is required to determine whether it may increase the volume of transmembrane flow compared to bupivacaine.
In our study, the data also suggested that a less concentrated local anesthetic (ropivacaine 0.08%) could be effective after DPE + PIEB for epidural catheter dosing without compromising motor function. Both DPE + PIEB and CEI + PIEB techniques had demonstrated similar levels of motor block and bilateral sensory levels. There was no difference between the two groups in terms of the Bromage score. Although, some studies have reported that compared to CEI, the PIEB used to maintain epidural analgesia may reduce the maternal motor block (15,19). However, we did not observe differences in the maternal motor blocks in this study. We thought that a low concentration of ropivacaine was used in this study and that a low concentration of ropivacaine produced a motor-sensory separation. In a previous study (20), the author used levobupivacaine, which is more effective than ropivacaine. Increasing the efficacy of anesthesia could lead to differences in the use of PIEB and CEI motor blocks in labor analgesia.
Our study showed that there was no significant difference in the incidence of vaginal delivery or side effects (such as postdural headache, itching, nausea, vomiting, maternal hypotension, and bradycardia) between the two groups. For DPE, this low side-effect profile was consistent with previous studies (11,12). The Apgar scores at 1 minute and 5 minutes after birth were both 10 points; neither group of fetuses was found to have bradycardia, indicating that these two analgesic techniques were safe for the mother and the fetus. Interestingly, this study found no difference in patient satisfaction between the groups, with an average satisfaction score of >9 points (for both groups). This suggested that, apart from the rapid onset of pain relief, other factors also determine adequate patient satisfaction.
This study has some limitations that should be noted. Firstly, we did not record the presence of contractions at the time of VAS data collection, and so it is difficult to precisely define the onset of labor analgesia when many women are compared at different delivery stages. Secondly, different programmed dose intervals alter the degree of difference in the observed effects (21). Further studies are needed to determine optimal PIEB settings when combined with DPE.
Conclusions
The percentage of parturients with adequate analgesia within 10 minutes of epidural bolus initiation from DPE + PIEB was higher than that from CEI + PIEB. DPE + PIEB provided a better analgesic effect during labor, a complete sacral block, and reduced local anesthetic consumption. Both models are similar in terms of their delivery mechanisms, maternal satisfaction, and side effects. DPE + PIEB technology is a novel approach to labor analgesia and is worthy of clinical application.
Acknowledgments
We thoroughly acknowledge the contribution by the participating doctors: Xiaofen Chen, Wenshui Yao, and Tuanfang Fang.
Funding: This study was supported by grants from the Fujian Provincial Maternity and Children’s Hospital Technology Innovation Project (grant No.YCXZ 18-24).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2281
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2281
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2281). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This is a prospective randomized cohort trial approved by the institutional ethics committee of Fujian Maternal and Child Health Hospital (Ethics No. 168 June 12, 2017) and adhered to the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carvalho B, George RB, Cobb B, et al. Implementation of Programmed Intermittent Epidural Bolus for the Maintenance of Labor Analgesia. Anesthesia And Analgesia 2016;123:965-71. [Crossref] [PubMed]
- Onuoha OC. Epidural Analgesia for Labor: Continuous Infusion Versus Programmed Intermittent Bolus. Anesthesiol Clin 2017;35:1-14. [Crossref] [PubMed]
- Mowat I, Tang R, Vaghadia H, et al. Epidural distribution of dye administered via an epidural catheter in a porcine model. Br J Anaesth 2016;116:277-81. [Crossref] [PubMed]
- Sng BL, Kwok SC, Sia ATH. Modern neuraxial labour analgesia. Curr Opin Anaesthesiol. 2015;28:285-9. [Crossref] [PubMed]
- Rodríguez-Campoó MB, Curto A, González M, et al. Patient intermittent epidural boluses (PIEB) plus very low continuous epidural infusion (CEI) versus patient-controlled epidural analgesia (PCEA) plus continuous epidural infusion (CEI) in primiparous labour: a randomized trial. J Clin Monit Comput 2019;33:879-85. [Crossref] [PubMed]
- Bullingham A, Liang S, Edmonds E, et al. Continuous epidural infusion vs programmed intermittent epidural bolus for labour analgesia: a prospective, controlled, before-and-after cohort study of labour outcomes. Br J Anaesth 2018;121:432-7. [Crossref] [PubMed]
- Wilson SH, Wolf BJ, Bingham K, et al. Labor Analgesia Onset With Dural Puncture Epidural Versus Traditional Epidural Using a 26-Gauge Whitacre Needle and 0.125% Bupivacaine Bolus: A Randomized Clinical Trial. Anesthesia And Analgesia 2018;126:545-51. [Crossref] [PubMed]
- Chau A, Bibbo C, Huang CC, et al. Dural Puncture Epidural Technique Improves Labor Analgesia Quality With Fewer Side Effects Compared With Epidural and Combined Spinal Epidural Techniques: A Randomized Clinical Trial. Anesthesia And Analgesia 2017;124:560-9. [Crossref] [PubMed]
- Heesen M, Rijs K, Rossaint R, et al. Dural puncture epidural versus conventional epidural block for labor analgesia: a systematic review of randomized controlled trials. Int J Obstet Anesth 2019;40:24-31. [Crossref] [PubMed]
- Narang A, Yadav P, Kumari I, et al. Comparison of dural puncture epidural technique versus conventional epidural technique for labor analgesia in primigravida. Journal of Obstetric Anaesthesia and Critical Care 2018;8:24-8. [Crossref]
- Cappiello E, O'Rourke N, Segal S, et al. A randomized trial of dural puncture epidural technique compared with the standard epidural technique for labor analgesia. Anesth Analg 2008;107:1646-51. [Crossref] [PubMed]
- Thomas JA, Pan PH, Harris LC, et al. Dural puncture with a 27-gauge Whitacre needle as part of a combined spinal-epidural technique does not improve labor epidural catheter function. Anesthesiology 2005;103:1046-51. [Crossref] [PubMed]
- Gibiino G, Distefano R, Camorcia M, et al. Maternal epidural pressure changes after programmed intermittent epidural bolus (PIEB) versus continuous epidural infusion (CEI). Eur J Anaesthesiol 2014;31:183-4. [Crossref]
- Wong CA, McCarthy RJ, Hewlett B. The effect of manipulation of the programmed intermittent bolus time interval and injection volume on total drug use for labor epidural analgesia: a randomized controlled trial. Anesth Analg 2011;112:904-11. [Crossref] [PubMed]
- Capogna G, Camorcia M, Stirparo S, et al. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesth Analg 2011;113:826-31. [Crossref] [PubMed]
- Bernards CM, Kopacz DJ, Michel MZ. Effect of needle puncture on morphine and lidocaine flux through the spinal meninges of the monkey in vitro. Implications for combined spinal-epidural anesthesia. Anesthesiology 1994;80:853-8. [Crossref] [PubMed]
- Duzenmas D. Are the conclusions supported by the statistics? Anesth Analg 2010;110:969-author reply 969. [Crossref] [PubMed]
- Clement R, Malinovsky JM, Le Corre P, et al. Cerebrospinal fluid bioavailability and pharmacokinetics of bupivacaine and lidocaine after intrathecal and epidural administrations in rabbits using microdialysis. J Pharmacol Exp Ther 1999;289:1015-21. [PubMed]
- Leone Roberti Maggiore U, Silanos R, Carlevaro S, et al. Programmed intermittent epidural bolus versus continuous epidural infusion for pain relief during termination of pregnancy: a prospective, double-blind, randomized trial. Int J Obstet Anesth 2016;25:37-44. [Crossref] [PubMed]
- Casati A, Putzu M. Bupivacaine, levobupivacaine and ropivacaine: are they clinically different? Best Pract Res Clin Anaesthesiol 2005;19:247-68. [Crossref] [PubMed]
- Epsztein Kanczuk M, Barrett NM, Arzola C, et al. Programmed Intermittent Epidural Bolus for Labor Analgesia During First Stage of Labor: A Biased-Coin Up-and-Down Sequential Allocation Trial to Determine the Optimum Interval Time Between Boluses of a Fixed Volume of 10 mL of Bupivacaine 0.0625% With Fentanyl 2 µg/mL. Anesth Analg 2017;124:537-41. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


