
Moxifloxacin is a safe and effective candidate agent for tuberculosis treatment: a meta-analysis of randomized controlled trials
Introduction
At present, the incidence of tuberculosis is slowly declining globally, but the total number of cases is still enormous and poses a significant challenge to the capacity of healthcare systems in many countries (1,2). Therefore, tuberculosis is still one of the major threats to global health, and greater efforts are needed to improve the effectiveness of treatment. At present, anti-tuberculosis therapy primarily relies on a combination of medications to reduce drug resistance (3).
Currently, the implementation of tuberculosis treatment programs involves the combined administration of isoniazid, rifampin, pyrazinamide, and ethambutol for 2 months, followed by combined treatment with isoniazid and rifampin for 4 months. To reduce drug resistance, a combination of two drugs (isoniazid and rifampin) and three drugs (isoniazid, rifampin, and pyrazinamide) are often recommended in clinical practice (4,5). However, due to long-term use, compliance is poor in many patients (6). Therefore, new drugs that shorten the treatment time for tuberculosis can greatly reduce the possibility of disease recurrence and death due to insufficient treatment.
The fluoroquinolone drug moxifloxacin has shown antibacterial activity against Mycobacterium tuberculosis in vitro and in vivo (7,8). Early studies of moxifloxacin in mouse models have demonstrated that moxifloxacin has good bactericidal activity and can replace isoniazid (9). Although the use of moxifloxacin as a supplement in the treatment of tuberculosis has shown good results, the results of many clinical randomized controlled trials (RCTs) published in recent years are not completely consistent with each other. In order to determine the role of moxifloxacin in the treatment of tuberculosis, a meta-analysis of all available studies was conducted to comprehensively evaluate the efficacy and safety of adding moxifloxacin for the treatment of tuberculosis.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2612).
Methods
Literature search
We searched numerous databases, including PubMed, Embase, the Cochrane Controlled Center Register of Controlled Trials (CENTRAL), Web of Science, Baidu Scholar, and Google Scholar, from the date of inception of the database to September 25, 2020, without language restrictions. The search strategy was formulated with reference to the Cochrane Handbook. The English keywords were moxifloxacin and tuberculosis.
Inclusion and exclusion criteria
Inclusion criteria: (I) study type was RCT; (II) subjects were pulmonary tuberculosis patients over 18 years of age; (III) moxifloxacin was included in the treatment plan of the experimental group, and the treatment plan of the control group did not contain moxifloxacin; and (IV) the outcome indicators of the study were: (i) rate of sputum culture conversion, (ii) incidence of adverse reactions, (iii) incidence of serious adverse reactions, (iv) mortality, and (v) recurrence rate.
Exclusion criteria: (I) articles that did not meet the inclusion criteria; (II) the main outcomes of the article could not be obtained, including those cases where we did not receive a response from the author; and (III) repeatedly published articles.
Information and data extraction
We obtained the general characteristics of the included studies by reading the full texts, as well as by examining the inclusion criteria, basic data of the research subjects, intervention measures, follow-up time, main results, etc. For data that could not be obtained, we contacted the author/s as much as possible through email. The data was read and extracted by two authors independently. Any inconsistencies or disagreements were resolved through discussion between the reviewers. If these still could not be resolved, the third author was consulted and made the final decision.
Literature quality evaluation
Two researchers independently evaluated the included literature in accordance with the Cochrane Handbook 5.1.0 quality evaluation standard. The quality evaluation standard includes the following seven aspects: (I) generation of random sequence; (II) hidden grouping; (III) blinding of investigators and participants; (IV) blinding of outcome measurers; (V) incomplete report of patients and outcome events; (VI) selective result report; (VII) other limitations. Each item is divided into three levels: low risk of bias, unclear, and high risk of bias.
Statistical method
Meta-analysis was performed using RevMan5.1 software officially provided by Cochrane. First, the chi-square test and I2 test were used to analyze the heterogeneity among the studies. If the homogeneity between studies was good (I2<50%, P>0.1), the fixed effects model was used; otherwise, the random effects model was employed. If the clinical data could not be meta-analyzed, a descriptive analysis was performed.
Results
Literature search results
In total, 4,384 articles were initially retrieved. Of these, 3,650 articles remained after using EndNote software (Thomson Corporation, Connecticut, USA) to eliminate duplicate articles. After reading the titles, abstracts, and full texts, 13 articles were finally included in the meta-analysis. The literature screening flow chart is shown in Figure 1.
General characteristics of included articles
A total of 13 studies (10-22) were included in this meta-analysis, all of which were designed as RCTs and involved 7,774 patients. The articles were published between 2006 and 2020. Patients in the articles mainly came from Africa, North America, and South America, mostly from Brazil, Uganda, India, Tanzania, Kenya, Thailand, Malaysia, Zambia, Spain, and China. The details of these studies are shown in Table 1.
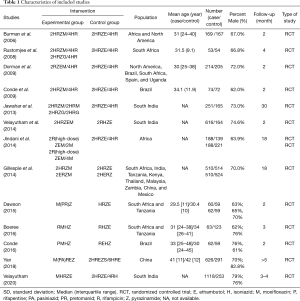
Full table
Quality evaluation
Figure 2 shows the risk of bias of the included RCTs, and the results were acceptable.
Meta-analysis results
Rate of sputum culture conversion
Thirteen studies analyzed the rate of sputum culture conversion in pulmonary tuberculosis patients 2 months after treatment with and without moxifloxacin. The results showed that the rate of sputum culture conversion of the experimental group was significantly better than that of the control group, and the difference was statistically significant [relative risk (RR) =1.12, 95% confidence interval (CI): 1.06–1.18, P<0.0001, see Figure 3).
Rate of adverse reactions
Twelve studies analyzed the incidence of adverse reactions in the treatment of tuberculosis patients with and without moxifloxacin. The results showed that the incidence of adverse reactions in the experimental group was significantly higher than that in the control group, and the difference was statistically significant (RR =1.34, 95% CI: 1.07–1.67, P=0.01, see Figure 4).
Incidence of serious adverse reactions
Twelve studies analyzed the incidence of serious adverse reactions in patients with pulmonary tuberculosis treated with and without moxifloxacin. The results showed that there was no significant difference in the incidence of serious adverse reactions between the experimental group and the control group (RR =1.09, 95% CI: 0.90–1.33, P=0.38, see Figure 5).
Mortality and recurrence rate
Nine studies analyzed the mortality of tuberculosis patients treated with and without moxifloxacin. The results showed that there was no statistically significant difference in mortality between the experimental group and the control group (RR =0.79, 95% CI: 0.51–1.21, P=0.28, Figure 6). Seven studies analyzed the recurrence rate of tuberculosis patients treated with and without moxifloxacin treatment regimens. There was no significant difference in the recurrence rate between the two groups (RR =1.41, 95% CI: 0.61–3.25, P=0.42, Figure 7).
Discussion
In addition to the new drugs recommended by the World Health Organization for the treatment of multidrug-resistant tuberculosis such as linezolid, bedaquinoline and dramanib, there are also about 17 new compounds targeting Mycobacterium tuberculosis that are currently undergoing different experimental phase of clinical trials. Moxifloxacin has demonstrated antibacterial activity against both gram-positive cocci and gram-negative bacteria, and especially against respiratory pathogens (23). Studies have shown that moxifloxacin is effective in the treatment of chronic bronchitis, skin infections, community-acquired pneumonia, and bacterial infections (24,25). In recent years, an increasing number of high-quality clinical studies have focused on the application of moxifloxacin in the treatment of tuberculosis (18,20). However, the latest evidence-based medicine research has not yet been updated.
This meta-analysis included a total of 13 qualified RCTs to evaluate the safety and efficacy of adding moxifloxacin to the treatment regimen of tuberculosis patients. The results showed that the RR value for the rate of sputum culture conversion was 1.12, and the difference was significant (P<0.0001), which indicates that the addition of moxifloxacin could improve the bactericidal activity of the treatment. In terms of overall adverse reactions, the addition of moxifloxacin could also significantly increase the incidence of adverse reactions. The RR value was 1.34, which is a significant difference (P=0.01), indicating that the introduction of moxifloxacin may increase adverse reactions during treatment. However, it did not increase the incidence of serious adverse reactions. In addition, the addition of moxifloxacin did not increase the mortality rate, and could not reduce the recurrence rate in the later period.
An earlier meta-analysis evaluated the clinical outcomes of moxifloxacin plus standard first-line treatment for tuberculosis (26). Patients in the control group were given a combination of pyrazinamide (Z), isoniazid (H), and rifampin (R), with or without ethambutol (Ethambutol, E). This was called standard therapy. Patients in the study group were given the standard regimen plus moxifloxacin. Six eligible studies were included. The results showed that compared with the control group, the addition of moxifloxacin did not increase the rate of sputum culture conversion in the study group, which is inconsistent with the results of our study. Moreover, the meta-analysis of Xu et al. (27) included nine appropriate studies, and the results showed that the introduction of moxifloxacin could improve the clinical efficacy by increasing the sputum culture conversion rate and reducing the recurrence rate. The ability of moxifloxacin to increase the sputum culture conversion rate is consistent with the results of our study. Earlier studies by Ruan et al. and Guan et al. also revealed similar results (28,29), however their results were mostly based on fewer studies or non-RCTs. Our present study included 13 qualified RCTs involving a greater number of patients than the aforementioned studies, and the results showed that the introduction of moxifloxacin into the treatment regimen did not reduce the recurrence rate of tuberculosis patients. The 13 RCTs included in this study all reported the rate of sputum culture conversion after 2 months of treatment. The overall data showed that the introduction of moxifloxacin in the early treatment regimen could significantly increase the bacteriostasis of the treatment regimen. Furthermore, the previous meta-analysis of Xu et al. involved only three studies that reported results related to the recurrence rate (27), and found that moxifloxacin could significantly reduce the recurrence rate. Meanwhile, in our meta-analysis, seven of the 13 RCTs included reported recurrence of sputum culture, and the results demonstrated that the introduction of moxifloxacin cannot reduce the recurrence rate. According to Chen et al., Whole Genome Sequencing (WGS) is a promising approach to predict resistance to H, R, Z, Levofloxacin with satisfactory accuracy, sensitivity, and specificity of over 85.0%. The specificity of WGS in diagnosing anti-tuberculosis drug resistance, and high-level resistance to moxifloxacine (2.0 mg/L) needs to be improved (30).
This study had some limitations that should be noted. Firstly, the follow-up time of the studies included in this meta-analysis varied, and the experimental group's medication regimen was not completely consistent, which may have affected the validity of our results. Also, due to the limited number of included studies, subgroup analysis was not performed.
Conclusions
This meta-analysis found that adding moxifloxacin to the treatment plan of tuberculosis patients could significantly increase the rate of sputum culture conversion after treatment; however, it has no significant effect on the recurrence rate. Also, the addition of moxifloxacin was found to increase the incidence of adverse reactions, but did not increase the incidence of mortality or serious adverse reactions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2612
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2612). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vashishtha VM. WHO Global Tuberculosis Control Report 2009: Tuberculosis elimination is a distant dream. Indian Pediatr 2009;46:401-2. [PubMed]
- Fonseca JD, Knight GM, McHugh TD. The complex evolution of antibiotic resistance in Mycobacterium tuberculosis. Int J Infect Dis 2015;32:94-100. [Crossref] [PubMed]
- Dobbs TE, Webb RM. Chemotherapy of Tuberculosis. Microbiol Spectr 2017;5. [Crossref] [PubMed]
- Tiberi S, du Plessis N, Walzl G, et al. Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect Dis 2018;18:e183-98. [Crossref] [PubMed]
- Zumla A, Nahid P, Cole ST. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov 2013;12:388-404. [Crossref] [PubMed]
- Dye C, Watt CJ, Bleed D. Low access to a highly effective therapy: a challenge for international tuberculosis control. Bull World Health Organ 2002;80:437-44. [PubMed]
- Ji B, Lounis N, Maslo C, et al. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother 1998;42:2066-9. [Crossref] [PubMed]
- Louie A, Duncanson B, Myrick J, et al. Activity of Moxifloxacin against Mycobacterium tuberculosis in Acid Phase and Nonreplicative-Persister Phenotype Phase in a Hollow-Fiber Infection Model. Antimicrob Agents Chemother 2018;62:e01470-18. [Crossref] [PubMed]
- Lounis N, Bentoucha A, Truffot-Pernot C, et al. Effectiveness of once-weekly rifapentine and moxifloxacin regimens against Mycobacterium tuberculosis in mice. Antimicrob Agents Chemother 2001;45:3482-6. [Crossref] [PubMed]
- Burman WJ, Goldberg S, Johnson JL, et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med 2006;174:331-8. [Crossref] [PubMed]
- Rustomjee R, Lienhardt C, Kanyok T, et al. Gatifloxacin for TBst. A Phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2008;12:128-38. [PubMed]
- Dorman SE, Johnson JL, Goldberg S, et al. Tuberculosis Trials C. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med 2009;180:273-80. [Crossref] [PubMed]
- Conde MB, Efron A, Loredo C, et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet 2009;373:1183-9. [Crossref] [PubMed]
- Jawahar MS, Banurekha VV, Paramasivan CN, et al. Randomized clinical trial of thrice-weekly 4-month moxifloxacin or gatifloxacin containing regimens in the treatment of new sputum positive pulmonary tuberculosis patients. PLoS One 2013;8:e67030. [Crossref] [PubMed]
- Gillespie SH, Crook AM, McHugh TD, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014;371:1577-87. [Crossref] [PubMed]
- Jindani A, Harrison TS, Nunn AJ, et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med 2014;371:1599-608. [Crossref] [PubMed]
- Velayutham BV, Allaudeen IS, Sivaramakrishnan GN, et al. Sputum culture conversion with moxifloxacin-containing regimens in the treatment of patients with newly diagnosed sputum-positive pulmonary tuberculosis in South India. Clin Infect Dis 2014;59:e142-9. [Crossref] [PubMed]
- Dawson R, Diacon AH, Everitt D, et al. Efficiency and safety of the combination of moxifloxacin, pretomanid (PA-824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: a phase 2b, open-label, partly randomised trial in patients with drug-susceptible or drug-resistant pulmonary tuberculosis. Lancet 2015;385:1738-47. [Crossref] [PubMed]
- Conde MB, Mello FC, Duarte RS, et al. A Phase 2 Randomized Trial of a Rifapentine plus Moxifloxacin-Based Regimen for Treatment of Pulmonary Tuberculosis. PLoS One 2016;11:e0154778. [Crossref] [PubMed]
- Boeree MJ, Heinrich N, Aarnoutse R, et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017;17:39-49. [Crossref] [PubMed]
- Yan L, Kan X, Zhu L, et al. Short-course Regimen for Subsequent Treatment of Pulmonary Tuberculosis: A Prospective, Randomized, Controlled Multicenter Clinical Trial in China. Clin Ther 2018;40:440-9. [Crossref] [PubMed]
- Velayutham B, Jawahar MS, Nair D, et al. 4-month moxifloxacin containing regimens in the treatment of patients with sputum-positive pulmonary tuberculosis in South India - a randomised clinical trial. Trop Med Int Health 2020;25:483-95. [Crossref] [PubMed]
- Simoens S. Evidence for moxifloxacin in community-acquired pneumonia: the impact of pharmaco-economic considerations on guidelines. Curr Med Res Opin 2009;25:2447-57. [Crossref] [PubMed]
- Mu YP, Liu RL, Wang LQ, et al. Moxifloxacin monotherapy for treatment of complicated intra-abdominal infections: a meta-analysis of randomised controlled trials. Int J Clin Pract 2012;66:210-7. [Crossref] [PubMed]
- Keating GM, Scott LJ. Moxifloxacin: a review of its use in the management of bacterial infections. Drugs 2004;64:2347-77. [Crossref] [PubMed]
- Chen Z, Liang JQ, Wang JH, et al. Moxifloxacin plus standard first-line therapy in the treatment of pulmonary tuberculosis: A meta-analysis. Tuberculosis (Edinb) 2015;95:490-6. [Crossref] [PubMed]
- Xu P, Chen H, Xu J, et al. Moxifloxacin is an effective and safe candidate agent for tuberculosis treatment: a meta-analysis. Int J Infect Dis 2017;60:35-41. [Crossref] [PubMed]
- Ruan Q, Liu Q, Sun F, et al. Moxifloxacin and gatifloxacin for initial therapy of tuberculosis: a meta-analysis of randomized clinical trials. Emerg Microbes Infect 2016;5:e12. [Crossref] [PubMed]
- Guan Y, Liu Y. Meta-analysis on Effectiveness and Safety of Moxifloxacin in Treatment of Multidrug Resistant Tuberculosis in Adults. Medicine (Baltimore) 2020;99:e20648. [Crossref] [PubMed]
- Chen X, He G, Wang S, et al. Evaluation of Whole-Genome Sequence Method to Diagnose Resistance of 13 Anti-tuberculosis Drugs and Characterize Resistance Genes in Clinical Multi-Drug Resistance Mycobacterium tuberculosis Isolates From China. Front Microbiol 2019;10:1741. [Crossref] [PubMed]
(English Language Editor: A. Kassem)









