
Bevacizumab as a treatment for radiation necrosis following stereotactic radiosurgery for brain metastases: clinical and radiation dosimetric impacts
Introduction
Brain metastases represent the most common malignant tumors in the central nervous system, which are an increasing challenge to modern oncology (1). The local treatment approaches for brain metastases are very limited including surgery, stereotactic radiosurgery (SRS) and whole-brain radiotherapy (WBRT) (2). SRS is a common radiotherapy option for brain metastases. It is cost-effective and well-tolerated for patients with brain metastases. However, around 10% of patients could develop radiation induced brain necrosis (RN) after SRS treatment, leading to progressive neurological impairment (3). Sequela of brain necrosis (BN) includes vascular injury, glial injury, neuronal injury, enzymatic disturbance and inflammatory response (4).
A growing body of evidence suggests that vascular endothelial growth factor (VEGF) is an essential factor in developing radiation BN (5). Bevacizumab is a humanized monoclonal antibody targeting VEGF. Anti-angiogenesis treatments have proved to be effective in management of RN. Therefore, Bevacizumab is used to treat RN due to the biological rationales of anti-VEGF. Corticosteroids had long been applied in RN treatment, however long-term use of corticosteroids is associated with substantial adverse effects (6). Bevacizumab has been confirmed to effectively reduce the use of corticosteroids in many preliminary studies (7-11). Of note, these preliminary studies were conducted on small sample sizes. The optimal dose, mode, and duration of Bevacizumab use have not been identified. The purpose of this study is to investigate the effects of Bevacizumab as a treatment for RN following SRS for brain metastases and to identify the clinical and radiation dosimetric factors that influence the efficacy. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2417).
Methods
Patients
From April 2011 to November 2019, a total 40 patients who were diagnosed with RN after SRS for brain metastases and subsequently treated with Bevacizumab (Avastin, Genentech Inc., South San Francisco, CA, USA) at our hospital were retrospectively analyzed. Eligibility criteria for inclusion are described as follows: (I) diagnosed with brain metastases; (II) whose radiographic data showed RN; (III) whose prior radiotherapy for brain metastases was performed ≥6 months before Bevacizumab treatment; (IV) received Bevacizumab 5 mg/kg intravenously once every two weeks or 10 mg/kg once every three weeks after diagnosed as BN and completed the course during follow-ups for 6 months. This retrospective study was approved by the institutional review board. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Diagnostic methods
The diagnosis of RN was based on the following characteristics: (I) the patient received high-dose, single, or fractional SRS with or without whole-brain metastases (WBRT); (II) progression of contrast-enhancing lesions was detected within the high-doses SRS fields; (III) the imaging features of the lesions were based on traditional imaging features such as “Swiss cheese“ and “soap bubble” appearance, as well as functional imaging support, such as hypoperfusion on dynamic susceptibility contrast MRI perfusion (4,12,13). The positron emission CT (18F-FDG PET/CT) of 18-F deoxyglucose tracer detected low glucose uptake in the lesion.
Data collection
Clinical data collected included age, gender, primary tumor condition, the dose and fractions of radiation, biologically effective doses (BED), duration between radiotherapy and RN diagnosis, Karnofsky Performance Status (KPS) before and after Bevacizumab administration, the time and dosages of corticosteroids, time for half-reduction dose of corticosteroids (or hormone halving time), the number of courses and dosages of Bevacizumab. We also evaluated gross tumor volume and kinetics during the treatments based on contrast-enhanced T1-weighted magnetic resonance imaging (MRI) and T2-weighted fluid-attenuated inversion recovery T2/FLAIR at different time points, i.e., prior to Bevacizumab treatment (V0T1 + C V0T2), 1 month (V1T1 + C, V1T2), 3 month (V3T1 + C, V3T2) and 6 month (V6T1 + C, V6T2) following the treatment. For quantitative evaluation, the MRI images were imported into the iPlan RT planning system (version 4.1, Brainlab AG), and the lesions were manually delineated by radiation oncologists and the volumes were therefore determined.
Statistical analysis
Chi-square (χ2) statistics or Fisher’s exact tests were used to compare categorical variables. Student’s t-test was used to compare normally distributed variables. Correlation analysis was performed using Spearman’s correlation (2-tailed) and scatter plot analysis to analyze the correlations between the duration between SRS and necrosis diagnosis, the single dose in every course of Bevacizumab, the relative edema volume alternations before and after Bevacizumab, the edema volume reduction ratio [ΔVT2= (V1T2 − V1T2)/V1T2] and the index of BED × GTV as well as time for half reduction dose of corticosteroids. Chi-square (χ2) statistics or Fisher’s exact tests were performed using Stata (version 11.1, StataCorp LP, College Station, TX). Correlation analysis was performed using SigmaPlot v14.0 (Systat software Inc.) and GraphPad Prism v8.0.2 (GraphPad Software Inc.). All of the statistical assessments were two sided and P<0.05 was considered as statistically significant.
Results
Patient characteristics
The clinical characteristics of 40 brain metastases patients with RN were provided (Table 1). MRI images of representative RN response to Bevacizumab were shown (Figure 1). A total of 40 patients who received SRS for brain metastasis were enrolled in the present study. Fifteen patients received both whole-brain radiation therapy (WBRT) and SRS. The median latency period from the end of radiotherapy to RN diagnosis was 11 months (range, 7–35 months). There was no significant correlation between latency from the end of radiotherapy to the diagnosis of RN and a variety of clinical features such as age, gender, primary tumor, with or without the combination of WBRT, or the combination of targeted therapy such as EGFR TKI. The median VT2/VT1 + C ratio was 5.34 (range, 1.4–24) when RN was diagnosed. The initial V0T1 + C, V0T2 are listed in Table 2.
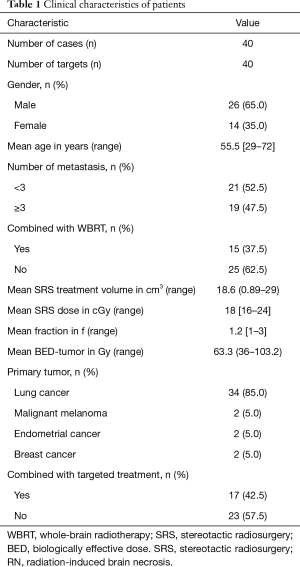
Full table
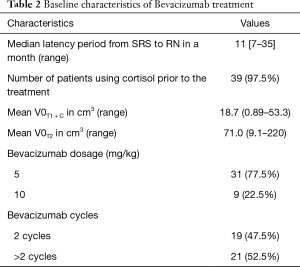
Full table
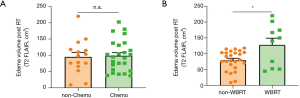
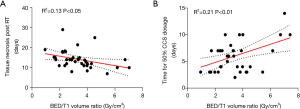
No significant difference was found in the edema volume (V0T2) between the chemotherapy group and non-chemotherapy group (P>0.05). Patients received with SRS + WBRT exhibited relatively larger edema volumes derived from T2 FLAIR post radiotherapy than those without WBRT (P<0.05) (Figure 2). Interestingly, the time between the end of radiotherapy and the onset of brain necrosis was found to be correlated negatively with the ratio of BED/GTV (Gy/cm3) (r2=0.13, P<0.05) (Figure 3A). In similar, as a surrogate for the severity of brain necrosis induced by radiotherapy, the time for half-reduction dose of corticosteroids (time for 50% CCS dosage) was correlated well with the ratio of BED/GTV (Gy/cm3) (r2=0.21, P<0.01) (Figure 3B).
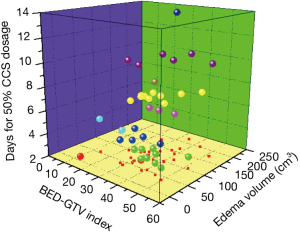
For a better evaluation of patients for radiation-induced brain necrosis, we next to establish a three-dimensional stratification on the basis of three critical parameters: the severity of the brain necrosis (days for 50% CCS Dosage), the volume of the brain necrosis (edema volume) as well as the radiation dose volume effect (BED × GTV) (Gy·cm3) (Figure 4). As a result, a higher BED × GTV index is associated with a shorter interval between radiotherapy and the onset of brain necrosis, and apparently larger edema volume, implicating an application of Bevacizumab.
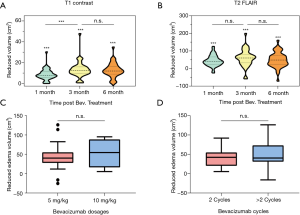
Radiographic response to Bevacizumab treatment
Among these 40 patients, T1 volume decreased to 38 patients after Bevacizumab treatment, and edema volume decreased in 38 patients, with a response rate of 95%. When compared with the tumor volume prior to Bevacizumab treatment (V0T1 + C), there was considerable reduction of tumor volume at 1 month (V1T1 + c), 3 months (V3T1 + c) and 6 months (V6T1 + C) post Bevacizumab treatment (all P<0.001) (Figure 5A). The mean reduction rate for 1-, 3- and 6-month were 48.4%, 74% and 75%, respectively (all P<0.001) (Table 2). Furthermore, in term of brain edema, remarkable shrink was observed at 1-month (V1T2), 3-month (V3T2) and 6-month (V6T2) post Bevacizumab treatment (all P<0.001) (Figure 5B). No significant difference was found in mitigating edema between the two difference dose regimens of Bevacizumab, i.e., 5 vs. 10 mg/kg (P>0.05), (Figure 5C). In similar, there was no significant difference in attenuating edema between the application of 2 vs. >2 cycles of Bevacizumab (P>0.05) (Figure 5D).
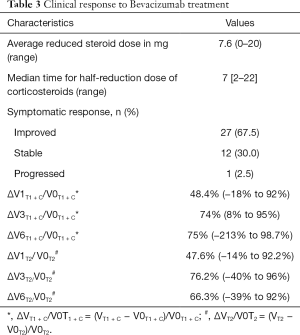
Clinical response to Bevacizumab treatment
After Bevacizumab treatment, the use of corticosteroid and the dosage of dehydration drugs were reduced. The time for half-reduction dose of corticosteroids (hormone halving time) were seven days (Table 3). The median KPS score after treatment was 70 (range, 60–90) compared much lower level as 50 (range, 40–80) prior to Bevacizumab treatment (P<0.001). No adverse events such as hypertension, proteinuria, and intracranial hemorrhage were observed during the entire course pf treatment with Bevacizumab.
Full table
Discussion
Radiation brain injury is a serious adverse event of radiotherapy. Brain tissue damage caused by radiotherapy can be divided into early reactions (which can occur during the radiotherapy period), early delayed reactions (occurring about 3 months after radiotherapy), and late radiation reactions (occurring several months to several years after radiotherapy) (14). Nowadays, SRS is a common approach for brain metastasis as SRS achieves good control in metastatic brain tumors (6). Unfortunately, SRS leads to RN in 10% of patients (3). The increasing incidence of RN has become a limiting factor of SRS prescription dose. The development of brain RN is related to a variety of factors including doses, fractionation, treated volume, combined chemotherapy, targeted therapy, and patients inherent factors (15). Rare studies are available so far to study the confounding factors of the occurrence speed and the severity of RN. The time course from the end of radiotherapy to the onset of RN reflects the occurrence speed of RN. It might be much accelerated when patients received SRS rather than conventional radiotherapy (16). The volume of a single lesion, or the total volume of multiple lesions, is one of the critical determinants for RN (15). We found that the ratio of BED/GTV (Gy/cm3) correlated positively with the time for half-reduction dose of corticosteroids, and negatively with the time course for development of radiation-induced brain necrosis. We also proposed a new radiation dose volume index, BED×GTV (Gy·cm3), which was found to facilitate the risk stratifications of patients for radiation-induced brain necrosis.
Compared with the incidence of radiographic change (46%), the incidence of symptomatic RN occurrence (14%) was relatively lower (17). The main purpose of treatment for brain metastasis patients with short life expectancy are to alleviate symptoms and improve life quality. Corticosteroids are commonly used to reduce brain edema caused by RN. However, corticosteroids have much side effects involving in multiple organs (4). Furthermore, corticosteroid refractory brain edema with serious neurological symptoms also highlights the need for additional therapeutic options. Bevacizumab, an anti-VEGF antibody, was firstly reported as a treatment for RN by Gonzalez et al. (6). Increasing reports have confirmed the effectiveness of Bevacizumab for RN in improving radiological and clinical manifestations such as KPS and clinical symptoms. The side effects for Bevacizumab are usually mild and with rare grade 3 or above toxicities (7-11).
In previous studies, Bevacizumab significantly reduced the volume of lesions of contrast-enhanced T1-weighted MRI (T1 + C) and T2 FLAIR. The percentage of reduced volume on T1 + C varied from 48–100%, and the percentage of reduced volume on T2 FLAIR varies from 49–66% (10). In previous studies, a variety of doses and duration of Bevacizumab were utilized. The common dosage of Bevacizumab was 5–7.5 mg/kg every 2 weeks or 10 mg/kg every three weeks. No significant radiographic improvements were observed with enhanced doses of Bevacizumab. It was reported that the number of Bevacizumab was mostly two to four cycles, and no significant radiographic improvements were detected with higher doses of Bevacizumab (10). Our results confirm that Bevacizumab treatment can significantly reduce the enhanced lesion volume and edema. A low dose (5 mg/kg) and short courses (two cycles) of treatment with Bevacizumab was found to ensure this effect. The improvement in radiographic in both enhancement and edema has the possibility of RN recurrence.
In our study, the volumes of most metastatic lesions were reduced to a minimized level at the 3-month of follow-up (V3T1 + C, V3T2). Some lesions (4/40, 10%) tended to prolong the time for the follow-up. Many other studies have suggested that the recurrence of BN after withdrawal of Bevacizumab (7-11). The recurrence was considered to be related to excessive vessels pruning caused by excessive Bevacizumab treatment, thereby aggravating the ischemia and hypoxia in the original BN area and exacerbating tissue necrosis (18). Despite of the control of brain metastases after radiotherapy, there remains a risk of local relapses. When follow-up time is prolonged, patients may have clinical events due to the primary lesion. During the 6-month follow-up period, 10% of the patients exhibited radiological progress, and it is difficult to distinguish between the RN recurrence and primary brain metastasis progressions. Few studies have focused on treatment after RN recurrence. Further, as the pathological basis of necrosis remains, new vessels will operate reactively around the necrosis area, and little can be done to alter this pathological process (19).
Indications for Bevacizumab treatment of RN are dependent on the radiological evidence, such as T1 + C, T2 FLAIR, T2/T1 ratio, functional MRI and PET/CT or PET/MR. A multi-modalities imaging are used to ensure high specificity and sensitivity, allowing the differentiated diagnosis of RN and tumor lesions (3,4,20). Few studies can accurately distinguish RN from tumor histology. It was reported that RN was in only 36 of 148 patients (24%) with high-grade glioma. In this cohort, 20 patients had pure RN, and 16 patients had mixed histology. Due to the anti-tumor effect on many sorts of malignancies, Bevacizumab is therefore recommended as the initial treatment for the cases of radiologically proved brain necrosis.
There are several limitations of the study. This is a single-center and retrospective study, and due to the relatively small sample size of our study, a large-scale study is warranted to validate our findings.
In conclusion, Bevacizumab is a favorable salvage treatment of BN after SRS for patients with BM. The effectiveness is mainly manifested in the radiological and symptom improvement. The development of RN was found to be largely associated with radiation dose and gross tumor volume, and hence we proposed two new indexes, i.e., BED/GTV (Gy/cm3) for quantitative assessment the severity and latency time, and BED×GTV (Gy·cm3) for risk stratifications. A low dose with two cycles of Bevacizumab is recommended. Our study has a potential to facilitate more individualized management of BN following SRS for brain metastases.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (NSFC) (No. 81703166), Natural Science Foundation of Guangdong Province (No. 2019A1515011943), China Postdoctoral Science Foundation (No. 2019M662974) and Science and Technology Program of Guangzhou (No. 202002030445), and Medical Scientific Research Foundation of Guangdong Province (A2020505 and A2020499). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2417
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2417
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2417). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the institutional review board. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Upadhyay UM, Tyler B, Patta Y, et al. Intracranial microcapsule chemotherapy delivery for the localized treatment of rodent metastatic breast adenocarcinoma in the brain. Proc Natl Acad Sci U S A 2014;111:16071-6. [Crossref] [PubMed]
- Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895-902. [Crossref] [PubMed]
- Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist 2003;9:180-8. [Crossref] [PubMed]
- Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000;217:377-84. [Crossref] [PubMed]
- Miyatake S, Nonoguchi N, Furuse M, et al. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo) 2015;55:50-9. [Crossref]
- Gonzalez J, Kumar AJ, Conrad CA, et al. Effect of Bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 2007;67:323-6. [Crossref] [PubMed]
- Torcuator R, Zuniga R, Mohan YS, et al. Initial experience with Bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol 2009;94:63-8. [Crossref] [PubMed]
- Wang Y, Pan L, Sheng X, et al. Reversal of cerebral radiation necrosis with Bevacizumab treatment in 17 Chinese patients. Eur J Med Res 2012;17:25. [Crossref] [PubMed]
- Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of Bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 2011;79:1487-95. [Crossref] [PubMed]
- Sadraei NH, Dahiya S, Chao ST, et al. Treatment of cerebral radiation necrosis with Bevacizumab: the Cleveland clinic experience. Am J Clin Oncol 2015;38:304. [Crossref] [PubMed]
- Zhuang H, Yuan X, Zheng Y, et al. A study on the evaluation method and recent clinical efficacy of Bevacizumab on the treatment of radiation cerebral necrosis. Sci Rep 2016;6:24364. [Crossref] [PubMed]
- Mullins ME, Barest GD, Schaefer PW, et al. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol 2005;26:1967-72. [PubMed]
- Barajas RF, Chang JS, Sneed PK, et al. Distinguishing Recurrent Intra-Axial Metastatic Tumor from Radiation Necrosis Following Gamma Knife Radiosurgery Using Dynamic Susceptibility-Weighted Contrast-Enhanced Perfusion MR Imaging. AJNR Am J Neuroradiol 2009;30:367-72. [Crossref] [PubMed]
- Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res 2000;153:357-70. [Crossref] [PubMed]
- Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 2011;6:48. [Crossref] [PubMed]
- Ruben JD, Dally M, Bailey M, et al. Cerebral radiation necrosis: Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 2006;65:499-508. [Crossref] [PubMed]
- Blonigen BJ, Steinmetz RD, Levin L, et al. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2010;77:996-1001. [Crossref] [PubMed]
- Delishaj D, Ursino S, Pasqualetti F, et al. Bevacizumab for the treatment of radiation-induced cerebral necrosis: a systematic review of the literature. J Clin Med Res 2017;9:273-80. [Crossref] [PubMed]
- Zhuang H, Shi S, Yuan Z, et al. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer 2019;18:21. [Crossref] [PubMed]
- Caroline I, Rosenthal MA. Imaging modalities in high-grade gliomas: Pseudoprogression, recurrence, or necrosis? J Clin Neurosci 2012;19:633-7. [Crossref] [PubMed]
(English Language Editors: L. Gray and J. Chapnick)


