
Prognostic nomogram for patients with lung metastatic renal cell carcinoma: a SEER-based study
Introduction
Renal cell carcinoma (RCC) was the most common renal parenchyma malignant that originated from the kidney, accounting for 80–85% of renal tumors and 2–3% of systemic malignant malignancies (1). According to statistics, the number of newly diagnosed cases of RCC worldwide in 2018 was 403,262, and the number of deaths were approximately 175,100 (2). Due to the lack of reliable diagnostic biomarkers and concealed early clinical symptoms of RCC, 20–30% of patients were in the advanced stage at the first visit (3,4). Even after surgical treatment with early-stage RCC, 30% of the patients progressed to metastatic RCC eventually (5,6).
RCC with distant metastasis at the time of diagnosis or metastasis after operation was collectively referred to as metastatic RCC (RCC). Cytoreductive nephrectomy (CN) was an aggressive treatment modality for patients with metastatic RCC. Metastatic RCC had a high mortality rate with a 5-year survival rate of approximately 10% and a median survival time of 13.9 months among patients treated with CN (7,8). In RCC organ metastasis, lung metastasis were common, followed by bone, but liver and brain metastasis remained relatively rare (9). Abdel-Rahman analysis of 5,992 patients with organ metastatic RCC found that the prognosis of bone metastasis was the best and while liver metastasis had the worst prognosis (10). The treatment of metastatic RCC was still a major clinical challenge. Therefore, it is necessary to further study the predictors of long-term survival in patients with metastatic RCC
At present, some studies had shown that sex, age, tumor size and other clinicopathological factors may affect the prognosis of patients with RCC. But there were still few studies on independent prognostic factors of lung metastatic RCC (11,12). Therefore, a comprehensive prognostic assessment model incorporating a variety of important demographic and clinicopathological variables was necessary. The nomogram based on the regression coefficients of each variable, combines many important prognostic correlates to more accurately predict individual patient survival rates (13).
In this study, we obtained prognostic factors and demographic features of patients with lung metastatic RCC from the Surveillance, Epidemiology, and End Results (SEER) database. We first used multivariate Cox regression analysis to identify independent prognostic risk factors, and then further constructed and verified the prognostic nomogram for patients with lung metastatic RCC based on the above results.
We present the following article in accordance with the TRIPOD reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1488).
Methods
Patients selection
In this study, all patients were extracted from the National Cancer Institute-funded SEER database. The SEER database contains approximately 28% US population, including patient demographic and clinical information, such as diagnosis year, sex, age, race, marital status, insurance, primary site, tumor grade, Tumor-Node-Metastasis (TNM) stage, histological type, treatment methods, cause of death and survival time (14). In addition, SEER database information on lung, liver, bone, and brain organ metastases is presented only for data from 2010 onwards. This study used the SEER*Stat software (version 8.3.6). Using the “Primary Site-labeled” variable, we identified 100,813 RCC patients between January 1, 2010 and December 31, 2016.
Exclusion criteria in our study were as follows (Figure 1): (I) not only one primary tumor (n=29,398); (II) unknown survival time (n=848); (III) diagnosed at 2016 (n=11,250); (IV) without or with unknown lung metastases (n=53,560); (V) with bone metastases (n=1,954); (VI) with brain metastases (n=428); (VII) with liver metastases (n=684); (VIII) T0 or T stage unknown (n=508); (IX) N stage unknown (n=141); (X) not systemic therapy (n=459); (XI) tumor size unknown; (XII) patients under 18 years (n=1).
Cases, which were diagnosed after January 1, 2016 were excluded to make sure that all cases had a longer than 1-year survival status observation at the last follow-up in December 2016. Finally, we confirmed the data of 1,563 eligible patients diagnosed with lung metastatic RCC. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study variables
Specific information on age, year of diagnosis, race, sex, tumor T-stage, N-stage, histology, tumor size, chemotherapy, surgery, radiotherapy, survival time and causes of death can be found in the SEER database. The overall survival (OS) time was defined as the time from the date of diagnosis of RCC to the date of death from any cause or the last follow-up visit in December 2016. Deaths from other causes were excluded from the analysis of cancer-specific survival (CSS).
Statistical analysis
According to the optimal cutoff value generated by the X-tile software (Version 3.6.1), the age was trivially stratified (<65 years, 65–74 years and >74 years; Figure S1). By RStudio software, all patients were randomized in a ratio of 6:4 into a primary cohort and validation cohort. In the entire cohort, Kaplan-Meier curves were used to detect variables related to OS and CSS of lung metastatic RCC and differences between the curves were analyzed by the log-rank test. We used univariate and multivariate Cox models to analyze prognostic factors associated with lung metastasis RCC and to measure hazard ratios (HR) and 95% confidence intervals (CI).
Based on the results of the multivariate Cox proportional hazard regression model, the RStudio software was used to construct prognostic nomograms of RCC patients with lung metastasis to predict the OS and CSS probability of each patient. In addition, the calibration curves and consistency index (C-index) were used to evaluate the predictive performance of the nomograms, and to calibrate the predictive ability of the nomograms for 1-, 2- and 3-year OS and CSS. The value of C-index statistic ranged from 0.5 (non-discrimination) to 1 (perfect discrimination), and a higher value means a better prognostic model. All these assessments were performed by bootstrap with 1,000 resamples. Since there is no direct clinical explanation for the C-index, we analyzed the decision curve analysis (DCA), a new method for evaluating predictive models for net benefit assessment in terms of clinical outcomes (15). SPSS software (version 20.0) and RStudio software (Version 1.2.5033) were used for all statistical analyses. A P value of <0.05 (two-sided) was considered statistically significant.
Results
Demographic and clinicopathologic characteristics
From 2010 to 2015, a total of 1,563 eligible lung metastatic RCC patients were included in our study cohort, of which the primary cohort included 937 patients and the validation cohort included 626 patients. Table 1 showed the clinical and demographic characteristics of RCC patients with lung metastases. There were 1,121 (71.7%) males and 442 (28.3%) females in the entire cohort. The majority of patients were <65 years and white in both cohorts. The most common tumors were clear cell adenocarcinoma, T3 stage, N0 stage and tumor size <7 cm. In the whole cohort, the median follow-up data was 15.00 months, of which 580 patients died and 453 died of RCC. The OS rates of 1-, 2-, and 3-year were 60.6%, 39.6%, and 26.7% (Figure S2A), respectively, and the CSS rates of 1-, 2-, and 3-year were 62.3%, 44.2% and 30.9% (Figure S2B).
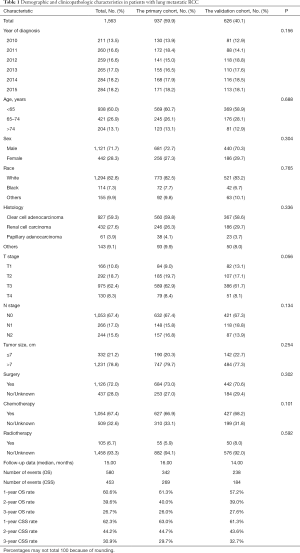
Full table
Identification of prognostic factors of OS and CSS in lung metastatic RCC patients
Univariate and multivariate Cox regression were used to analyze the prognostic factors associated with OS and CSS in patients with lung metastatic RCC. Univariate Cox regression analysis showed that histology, age at diagnosis, T-stage, N-stage, surgery, chemotherapy and radiotherapy were related factors of OS in patients with lung metastatic RCC in the primary cohort. After multivariate Cox regression analysis, only chemotherapy was not an independent risk factor for OS in patients with lung metastatic RCC (Table 2).
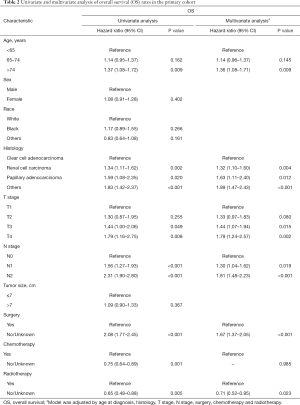
Full table
And in terms of CSS, in univariate Cox regression analysis the demographic and clinicopathological factors, which were significantly correlated with CSS were diagnostic age, histology, N-stage, T-stage, surgery, chemotherapy and radiotherapy. All the factors identified above were included in the multivariate Cox proportional hazard regression analysis, indicating that histology, N-stage, T-stage and surgery were independent prognostic factors of RCC with lung metastasis RCC, while age, chemotherapy and radiotherapy were not (Table 3).
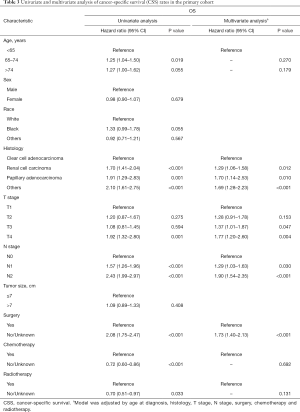
Full table
Prognostic nomograms for OS and CSS in patients with lung metastatic RCC
In the primary cohort, combined with the results of multivariate Cox regression analysis of OS and CSS in patients with lung metastatic RCC, we developed and established nomograms of OS and CSS that included all independent variables. Figure 2 shows the nomogram of the prognosis of 1-, 2- and 3-year OS and CSS.
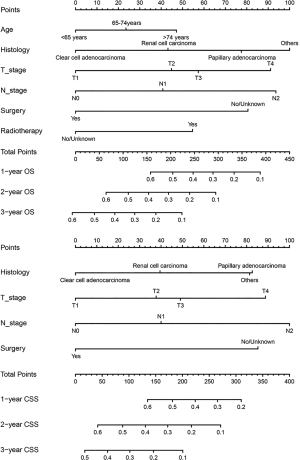
In the nomogram, the length of the line corresponding to each predictive variable represented its degree of effect on the survival outcome. No matter of the OS or CSS nomograms, surgery scores made the greatest contribution to the survival outcome, followed by chemotherapy, and marital status contributed the least to survival outcomes.
Validation and calibration of the nomograms
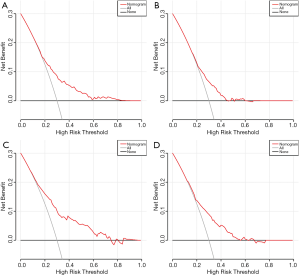
We used C-index and DCA curves to compare whether the survival times predicted by the nomograms were consistent with the actual survival times. In the primary cohort, the C-index of the nomogram OS was 0.662 (95% CI: 0.640–0.684) and the C-index of CSS was 0.658 (95% CI: 0.634–0.682). The C-index of the nomogram OS and CSS were 0.694 (95% CI: 0.670–0.719) and 0.685 (95% CI: 0.659–0.711), respectively in the validation cohort. Moreover, DCA curves also showed better clinical utility of the nomogram in both cohorts (Figure 3). This result indicated that the prognostic nomogram models we constructed was quite accurate.
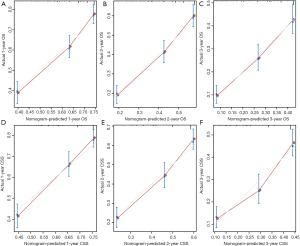
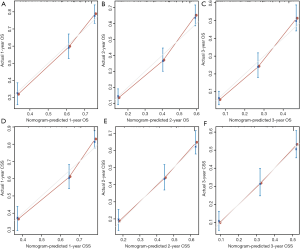
Moreover, we calibrated the 1-, 2- and 3-year OS and CSS nomogram of the primary and validation cohorts. In Figure 4 and Figure 5, the calibration curves were close to the perfect curves, which predicts a good consistency between nomogram predictions and the actual observation in the primary and validation cohorts.
Discussion
RCC was one of the common malignant tumors of the urinary system. In China, the incidence of RCC in 2015 was 66.8/100,000, and the mortality rate was 23.4/100,000 (16). In recent years, the incidence of RCC had been increasing. Compared with ten years ago, the incidence of RCC had increased by 2–3% (17). A considerable number of RCC patients had distant metastatic at the time of consultation, and their prognosis was poor. Among RCC organ metastatic, lung metastatic was the most common. However, the independent factors of lung metastasis RCC were still unknown. The purpose of our study was to determine independent prognostic indicators in patients with lung metastatic RCC.
In our study, Cox regression analysis was performed on a large number of patients with lung metastatic RCC from the SEER database. It was found that age at diagnosis, histology, N-stage, T-stage, surgery and chemotherapy were independent risk factors for OS and while histology, N-stage, T-stage and surgery were independent risk factors for CSS in patients with lung metastatic RCC. Based on the above result, we developed and established a prognostic nomogram of lung metastatic RCC patients. In addition, we calibrated and verified the above-constructed prognostic nomograms and evaluated the accuracy of the OS and CSS prognostic nomograms for 1-, 2- and 3-year. The results showed good agreement between nomogram predictions and the actual observations, and good reliability in both primary and external validation cohorts.
The nomogram was an image representation based on the results of multivariate survival regression analysis, which makes various prognostic factors more intuitive (18,19). The model integrates multiple independent prognostic survival factors, allowing for a more focused evaluation of individual patient survival probabilities (20). To date, prognostic nomograms have been developed for many cancer types and showed a more accurate ability to predict the prognosis than the traditional TNM systems (21). In addition, nomograms allowed clinicians to assess patients' physical condition more intuitively to provide personalized predictions. Therefore, the establishment of a reliable and effective prognostic nomogram is of great significance for the prognosis of patients with lung metastatic RCC and to provide individualized treatment.
The nomogram has been widely used in various tumors of the urinary system, and is important for accurate prognosis prediction and individualized treatment (22). Some studies have found that the bladder cancer nomogram was better than the prognostic model using standard pathological grouping, which can better predict the risk of recurrence after radical cystectomy (23). Similarly, Graefen et al. (24) also found the accuracy of the preoperative prostate nomogram prediction for prostate cancer. Zhang et al. (13) developed a nomogram for patients with clear cell renal cell carcinoma, which could accurately predict the incidence of 3-, 5-, and 10-year of OS and disease-specific survival (DSS) in these patients.
There were more and more studies on the nomogram of patients with distant organ metastatic. The most common was the study of bone metastatic tumors, but there were few studies on lung metastatic tumors. Hu et al. (25) studied 194 colorectal cancer patients with indeterminate pulmonary nodules and found that the clinical-radiomics nomogram based on radiological characteristics and clinical risk factors showed good discriminant ability and accuracy in predicting lung metastatic. To our knowledge, this was the first study of prognostic factors in patients with lung metastatic RCC, and reliable nomograms have been established. Using these nomograms, urologists could evaluate patients with lung metastatic RCC, enabling personalized treatment and monitoring possible.
There were some limitations to be recognized in the present study. First, the SEER database published information on liver, lung, bone, and brain-related organ metastases in 2010, follow-up was not long enough, and the degree and amount of lung metastasis were not available from the database. Second, the SEER database was a retrospective analysis and requires prospective studies for further validation. In addition, details of the type and duration of other adjuvant immunotherapies, targeted therapies, or systemic therapies were unclear. Moreover, information on patient complications or performance of metastasectomy was unclear.
Conclusions
Our study constructed a nomogram prognostic evaluation model for patients with lung metastatic RCC. The nomogram accurately and reliably predicted the 1-, 2- and 3-year OS and CSS of lung metastatic RCC.
Acknowledgments
We would like to thank three English experts, Jiatong Zhang, Dongyan Wang and Siwan Wang, for their language help.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81572517), Natural Science Foundation of Jiangsu Province (BK20161434), Jiangsu Provincial Medical Innovation Team (CXTDA2017025), National key research and development projects (SQ2017YFSF090096), and Jiangsu Provincial Medical Talent (ZDRCA2016080).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1488
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1488). The authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This article does not contain any studies with animals performed by any of the authors. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Matsuda T, Hori M. Five-year relative survival rate of kidney and renal pelvis cancer in the USA, Europe and Japan. Jpn J Clin Oncol 2015;45:136. [Crossref] [PubMed]
- Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016;70:93-105. [Crossref] [PubMed]
- Janzen NK, Kim HL, Figlin RA, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003;30:843-52. [Crossref] [PubMed]
- Kasenda B, Larkin J, Gore M. Immunotherapies in Early and Advanced Renal Cell Cancer. Prog Tumor Res 2015;42:1-10. [Crossref] [PubMed]
- Bhindi B, Abel EJ, Albiges L, et al. Systematic Review of the Role of Cytoreductive Nephrectomy in the Targeted Therapy Era and Beyond: An Individualized Approach to Metastatic Renal Cell Carcinoma. Eur Urol 2019;75:111-28. [Crossref] [PubMed]
- De Meerleer G, Khoo V, Escudier B, et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol 2014;15:e170-7. [Crossref] [PubMed]
- Chandrasekar T, Klaassen Z, Goldberg H, et al. Metastatic renal cell carcinoma: Patterns and predictors of metastases-A contemporary population-based series. Urol Oncol 2017;35:661.e7-661.e14. [Crossref] [PubMed]
- Abdel-Rahman O. Clinical correlates and prognostic value of different metastatic sites in metastatic renal cell carcinoma. Future Oncol 2017;13:1967-80. [Crossref] [PubMed]
- Wu J, Zhang P, Zhang G, et al. Renal cell carcinoma histological subtype distribution differs by age, gender, and tumor size in coastal Chinese patients. Oncotarget 2017;8:71797-804. [Crossref] [PubMed]
- Jung EJ, Lee HJ, Kwak C, et al. Young Age Is Independent Prognostic Factor for Cancer-Specific Survival of Low-Stage Clear Cell Renal Cell Carcinoma. Urology 2009;73:137-41. [Crossref] [PubMed]
- Zhang G, Wu Y, Zhang J, et al. Nomograms for predicting long-term overall survival and disease-specific survival of patients with clear cell renal cell carcinoma. Onco Targets Ther 2018;11:5535-44. [Crossref] [PubMed]
- Mao W, Zhang Z, Huang X, et al. Marital Status and Survival in Patients with Penile Cancer. J Cancer 2019;10:2661-9. [Crossref] [PubMed]
- Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA 2015;313:409-10. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Barata PC, Rini BI. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin 2017;67:507-24. [Crossref] [PubMed]
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Eastham JA, Kattan MW, Scardino PTJSUO. Nomograms as predictive models. Semin Urol Oncol 2002;20:108-15. [Crossref] [PubMed]
- Hou G, Zheng Y, Wei D, et al. Development and validation of a SEER-based prognostic nomogram for patients with bone metastatic prostate cancer. Medicine (Baltimore) 2019;98:e17197 [Crossref] [PubMed]
- Wu S, Chen JN, Zhang QW, et al. A New Metastatic Lymph Node Classification-based Survival Predicting Model in Patients With Small Bowel Adenocarcinoma: A Derivation and Validation Study. EBioMedicine 2018;32:134-41. [Crossref] [PubMed]
- Shariat SF, Karakiewicz PI, Suardi N, et al. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res 2008;14:4400-7. [Crossref] [PubMed]
- International Bladder Cancer Nomogram C. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006;24:3967-72. [Crossref] [PubMed]
- Graefen M, Karakiewicz PI, Cagiannos I, et al. International validation of a preoperative nomogram for prostate cancer recurrence after radical prostatectomy. J Clin Oncol 2002;20:3206-12. [Crossref] [PubMed]
- Hu , Wang S, Huang L, et al. A clinical-radiomics nomogram for the preoperative prediction of lung metastasis in colorectal cancer patients with indeterminate pulmonary nodules. Eur Radiol 2019;29:439-49. [Crossref] [PubMed]



