
Head and neck posture influences masticatory muscle electromyographic amplitude in healthy subjects and patients with temporomandibular disorder: a preliminary study
Introduction
Many clinical studies have assessed the relationship between head and neck posture and the craniomandibular complex (1-3). Head and neck posture may influence cervical vertebra bone changes, temporomandibular disorder (TMD) development, mandible growth and development, occlusion types, and breathing patterns (3). Recently, some studies have begun to investigate the important role of forward head posture (FHP) in the development of TMD (4-6). Different cervical postures may affect the mandibular position and trajectory, which may further affect the masticatory muscle function (7), forming a ‘skull-neck-jaw’ functional complex (8).
Recent studies have showed that chronic cervical pain and craniofacial pain are related to head and neck posture (9,10), and neck dysfunction is significantly related to mandibular dysfunction (11,12). Uritani et al. (13) found that the neck inclination angle of TMD patients was 7.1° lesser than that of healthy individuals through a comparative study between TMD patients and healthy individuals. This difference was not only statistically significant but also clinically significant, suggesting that the head position of TMD patients was more forward, but the association between head and cervical posture and TMDs is still controversial (3). There are also studies showed that postural alterations may be a risk factor for muscular TMD (4), while no significant changes in body posture were observed between subjects with and without unilateral disc displacement in the TMJ (14).
Ohmure et al. (2) determined that jaw muscle activity increases in the deliberate FHP compared with a natural head posture in healthy subjects, but TMD patients were not included in the study. Susan Armijo-Olivo (11) investigated the significance of cervical muscles in the development of TMD, and demonstrated no statistically significant differences in EMG activity of the anterior scalene or sternocleidomastoid muscles between TMD patients and healthy subjects. Furthermore, the EMG activity of masticatory muscles in TMD patients has been unclear due to the paucity of related research. It has been confirmed that there was a significant association between the sagittal head position and mandible position (15). However, the difference of masticatory EMG activity between healthy people and TMD patients under different head and neck postures has not yet been investigated.
The resting position of the mandible is described as a stable position relative to the maxilla (2–3 mm between the upper and lower incisors), which is maintained by the passive viscoelastic forces of the jaw supporting system and contractile elements and the muscle tone of the masticatory muscles (16). Different cervical postures may affect the mandibular position and trajectory, which may further affect the masticatory muscle function. During patient education in our department, a large number of patients with FHP complain that the resting position of the mandible is difficult to achieve. Although the patient’s teeth are not clenched, there is contact between the upper and lower incisors, this may affect the electrical activity of the masticatory muscles.
In this cross-sectional study, we hypothesized that the head and neck posture is different between TMD patients and healthy subjects, which would affect the basic electrical activities of the anterior temporal, masseter, superior trapezius, and sternocleidomastoid muscles. We used surface electromyography (sEMG) to measure the electrical activity of muscles in three different positions: (I) habitual relaxation position, (II) habitual relaxation position with tooth contact, and (III) mandibular resting posture with neutral head position (NHP). We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1850).
Methods
Study population
This is a cross-sectional study consisting of 16 TMD patients and 17 healthy subjects from July 2018 to February 2019.
The study conformed to the STROBE Statement and was approved by the Ethics Committee of Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (NO.: 2018-96-T87), Shanghai, China, based on the guidelines set forth in the Declaration of Helsinki (as revised in 2013, Approval date: 21/05/2018, Approval code: 2018-96-T87). All subjects provided informed consent for participation after receiving an explanation on the study procedures.
The healthy subjects had no neck and shoulder discomfort and TMD-related symptoms, and also met the inclusion criteria.
Inclusion criteria
(I) age between 18 and 75 years old; (II) diagnosis of myogenic TMD according to the diagnostic criteria for TMD (DC/TMD) (17); (III) presence of pain in the TMJ and its surroundings, and/or joint noise within 6 months; (IV) no other treatment, such as medication, physical therapy, and joint injection, in the past 2 weeks.
Exclusion criteria
(I) tooth loss, class II or class III malocclusion, sleep bruxism or orthodontic treatment history; (II) the visual analogue scale (VAS) score of pain for mastication ≥6, which affected the sEMG signal acquisition; (III) neck or shoulder pain and tenderness, which may affect the head and neck posture; (IV) fracture, joint sprain, and dislocation; posture disorder caused by abnormal curvature of spine; rheumatic disease; haemorrhagic disease, etc.; and (V) inflammatory, oncologic, or viral diseases of the face, jaw, spine, orskeletal muscles.
Image collection
The sagittal plane images of the upper body of each subject were taken using a digital camera in a habitual relaxation sitting position and NHP. Red markers were placed over the left eye edge, tragus, and C7 spinous process by experienced therapists with 4 years’ experience. The craniovertebral angle (CVA) is defined as the angle between the horizontal plane (the line perpendicular to true vertical axis) and the line extending from the tragus of the ear to C7 spinous process, which is highly reliable in assessing the forward head position, as previous studies proved (18,19). Measurement of the cervical angle was performed using a protractor, and the digitisation procedure was found to be highly reliable (intraclass correlation coefficient 0.98) (19). The cranial rotation angle is formed by a line connecting the lateral canthus and the tragus with a horizontal line (Figure 1) (13).
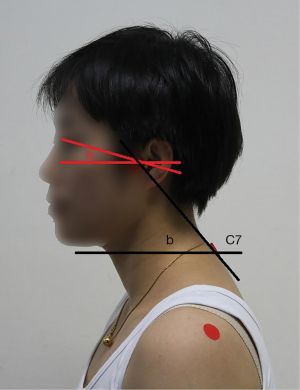
In order to ensure the consistency of the images taken, all the images of the subjects were taken by specially assigned personnel, who was blinded to the subject group. The distance between the camera and the subject was 1.5 m and the height was adjusted according to the subject to keep it level with the shoulder of the subject. After the therapist determined the body surface markers, the subjects took a comfortable habitual sitting position, the height of the chair was 45 cm, with the eyes focused at the front. Two images were taken for each subject to measure and calculate the parameters associated with posture.
The intra-rater and inter-rater reliability of head and neck posture measurement were evaluated in a previous study (13). Healthy participants were recruited, and photos were taken using the above method. Two raters evaluated independently by angulometer and the assessment were performed again after one month. The intra-rater reliability was 0.981, and inter-rater reliability was 0.980 for the angulometer assessment of FHP, which was has a good reliability in evaluating head and neck posture.
sEMG recordings and measurement
The masseter and anterior temporalis muscles, sternocleidomastoid muscle, and upper trapezius muscle of both sides (left and right) were examined. sEMG activity was recorded using a wireless electromyographic (EMG) system (Clinical DTS, 584-8C, Noraxon, USA), with light probes (weight, 5 g) clipped to the electrodes. The skin was polished by abrasive cloth and then scrubbed by a gauze pad soaked in 75% alcohol to reduce impedance. Disposable self-adhesive Ag/AgCl electrodes were placed on the skin just above the muscle belly and parallel to the direction of muscle fibres. The distance between the two electrodes was 2 cm (20). The sEMG signal sampling rate was set at 1,500 Hz, filtered through a band-pass of 10–500 Hz.
The maximal voluntary contraction (MVC) was measured to standardize the sEMG potentials of the four analysed muscles. The subjects were instructed to complete the MVC tests by an experienced therapist with 4 years’ experience, who was blinded to the subject group. Each action lasts for 5 s and repeated twice, with a 5-mininterval between each test.
- The masseter and anterior temporalis muscles: the subjects were seated and two 10-mm-thick cotton rolls were positioned between the upper and lower molars of both sides. The subjects were instructed to tightly bite the cotton roll as much as possible.
- Upper trapezius muscle: The subjects were seated with the head turned to the opposite side; the tester exerted resistance against the shoulder in the direction of depression and against the head in the direction of flexion anterolaterally. The subjects were asked to move the occiput toward the elevated shoulder with the face turned toward the opposite direction against resistance as much as possible.
- Sternocleidomastoid muscle and neck flexors: The subjects were placed in a supine position and the thorax was fixed, with the elbows bent and the hands overhead, resting on the table. The subjects were instructed to flex the cervical spine by lifting the head from the table, with the chin depressed and approximated toward the sternum. The testers applied resistance against the forehead in a posterior direction.
The 2-s period with the most stable signals was selected, and the corresponding mean value of each muscle’s root mean square (RMS) sequence was referred to as 100% of MVC amplitude.
Head positions
The basic EMG activities of the bilateral superior trapezius, sternocleidomastoid, anterior temporal, and masseter muscles were measured in different head positions. The subjects were seated comfortably in a chair (45-cm height).
The experiment protocol included three different positions.
- Habitual relaxation position: the subjects took a comfortable sitting position according to their daily habits, with eyes looking toward the front and breathing naturally, both feet on the ground and hands placed on top of their legs.
- Habitual relaxation position with tooth contact: on the basis of the first position, instructs the patient to look toward the front, relax naturally, and gently touch the upper and lower teeth without clenching.
Mandibular resting position with NHP: before taking photos, the subjects were given posture education to guide the key points of NHP and practice. The subjects sat against the wall, keeping the spine upright, using the wall as a vertical reference, with both hands placed on top of their legs and feet on the ground. The subjects were asked to adjust their heads to the neutral position, with the mandible naturally relaxed (21). NHP is readily determined by a lateral photograph to visually assess the alignment of tragus of the ear related to the midline of trunk (21).
After postural stabilisation, the mean value of three 10-s RMS segments was collected for 2 min, and the MVC ratio of the three segments to the corresponding muscle was used to express the muscle activation degree.
Clinical examination
All the subjects were examined by one physician using the DC/TMD international examination form. The physician was familiar with the DC/TMD with more than 5 years of experience in treating TMD patients. The duration of symptoms was 1–6 months. Specific questions were asked about the participant’s pain, and then the opening and closing of the mouth, vertical range of motion, and location of pain during opening were examined. Sixteen extraoral muscle sites and four intraoral muscle sites were palpated to assess for muscle pain, and two extraoral sites were palpated to assess for TMJ pain. The maximum painless mouth opening (mm) and visual analogue scale (VAS) score of pain were recorded.
Statistical methods
Statistical analysis was performed using SPSS Statistics for Windows, Version 23 (IBMCorp. Software, Armonk, NY, USA). The sample size calculation performed using PASS ver. 15 was based on an anticipated standard deviation of 0.5 and group difference of 0.4 according to the pre-experimental results. With a power of 80% and a one-sided α of 0.05, 13 participants were required per group. The measurement data were expressed as means ± standard deviation; the independent t-tests were used to evaluate the differences between the groups in terms of weight, height, age, and head-neck angle. Mixed analysis of variance was used to evaluate the effects of head posture on muscle electrical activity in each group. We reported the simple main effect when group-by-head posture interaction was significant, and the main effect when the interaction was not significant. Independent t-test was used to explore group differences at different head positions. The level of statistical significance for hypothesis testing was set at 0.05.
Results
In total, 17 healthy subjects (male: 3, female: 14) and 16 TMD patients (male: 3, female: 13) were included in this study. Of the 17 patients, 10 were bilateral, 6 were right and 1 was left. The head rotation angle and head-neck angle were used to reflect head and neck posture. The average head rotation angle of the healthy group was 20.31°±2.28° and that of the TMD group was 19.68°±4.96°. There was no significant difference between the two groups (P=0.549); the average CVA of the healthy group was 51.39°±4.75° and that of the TMD group was 45.46±5.23°. The difference between the two groups was 5.92° (95% CI: 3.43–8.40°), which was statistically significant (P<0.001). See Table 1 for specific values.

Full table
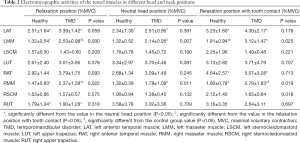
For the included participants in both groups, the EMG data of the tested muscles are shown in Table 2. We found no significant posture-by-group interaction for the left anterior temporal muscle (LAT) (F=1.120, P=0.314). The main effect of head posture was significant for LAT (F=9.557, P=0.002). In both groups, the basal RMSs of the LAT in the relaxation position and relaxation position with tooth contact were higher than that in the NHP, with significant differences (P<0.05) (Figure 2A). No significant group differences were found for the LAT. There was no significant posture-by-group interaction for the right anterior temporal muscle (RAT) (F=0.05, P=0.876). The group differences for the RAT were not significant. The main effect of head posture was significant (F=7.643, P=0.006). In both groups, the basal RMS of the RAT in the relaxation position with tooth contact was significantly higher than that in the NHP (P<0.05) (Figure 2B).
Full table
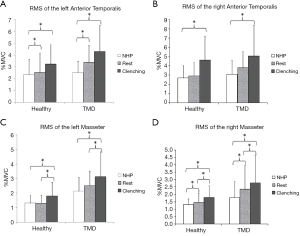
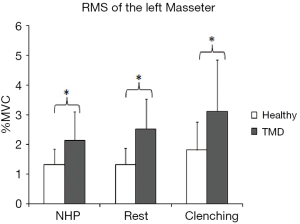
The main effect of head posture was significant (F=5.850, P=0.005) for the left masseter muscle (LMM) (no significant posture-by-group interaction). In both groups, the basal RMSs of the LMM in the relaxation position and NHP were lower than that in the relaxation position with tooth contact, with significant differences (P<0.05) (Figure 2C). There was no significant posture-by-group interaction for the right masseter muscle (RMM) (F=0.790, P=0.459). The main effect of head posture was significant (F=12.393, P<0.001) for the RMM. In both groups, the basal RMS of the RMM in the relaxation position with tooth contact was significantly higher than that in the relaxation position and NHP (P<0.05), and the basal RMS of the RMM in the relaxation position was also significantly higher than that in the NHP (P<0.05) (Figure 2D). We found significant group differences for RMM in the relaxation position (P=0.022), NHP (P=0.011), and relaxation position with tooth contact (P=0.019). The group differences for the LMM were significant in the relaxation position (P<0.001), NHP (P=0.007), and relaxation position with tooth contact (P=0.02) (Figure 3).
There was no significant difference between the groups for the left sternocleidomastoid muscle (LSCM) and right sternocleidomastoid muscle (RSCM) across the head positions (P>0.05). The main effect of head posture was not significant for the LSCM (F=1.920, P=0.167) and RSCM (F=1.954, P=0.162) (no significant group-by-angle interaction).
There was no significant posture-by-group interaction for the right upper trapezius (RUT) (F=0.513, P=0.559). No significant differences were found between the groups. The main effect of head posture was significant (F=8.574, P=0.002) for the RUT. In both groups, the basal RMSs of the RUT in the NHP were higher than that in the relaxation position, with significant difference (P<0.05).
Discussion
This study analysed the sEMG of the bilateral anterior temporalis, masseter, sternocleidomastoid, and upper trapezius muscles in the relaxation position, NHP, and relaxation position with tooth contact in TMD patients and compared the findings with those for healthy subjects. According to the results of this study, significant differences in muscle activity were found for the bilateral anterior temporalis muscles, bilateral masseter muscles, and RUT muscle across all head postures in the TMD and healthy groups. Significant group differences in the activity of bilateral masseter muscles were also found in this study.
Our study found that bilateral anterior temporalis muscle activity was significantly greater in the relaxation position with tooth contact than in the NHP. One explanation for this fluctuation is the influence of head position on the anterior temporalis muscle. The cranium, cervical spine, and mandible compose a vital unit called the ‘craniocervical-mandibular system’, and an altered head and neck posture may lead to orofacial pain, migraine, and cervical dysfunction (7). Piancino et al. (22) reported that the head posture affected the function of masticatory muscles and occlusal features. The head position tends to be more anterior when the participant maintains a relaxed position. A significantly posterior position of the condyle during FHP may result in an increase in the activity of the anterior temporalis muscle, relative to that observed with the condylar position in the NHP. The increased muscle activity attributed to the altered head and neck posture may contribute to pain in the surrounding muscles, chronic fatigue syndrome, and TMD development (2).
Sleep bruxism and daytime clenching have been considered as risk factors for TMD (23-25). Research findings suggested that the frequency of non-functional tooth contact was significantly higher in TMD patients than in healthy subjects (24), and occlusal abnormalities may prevent stable occlusion of the mandible (26,27). A significant increase in the electrical activity of the bilateral anterior temporalis muscles and masseters in the relaxation position with tooth contact was also demonstrated in the present study, which may amplify the degree of myofascial pain and lead to fatigue of the muscle in the long term. Thus, it is critical for physiotherapists to educate TMD patients on the importance of maintaining are laxed position, which aids in correcting clenching and reducing muscle activity.
An increasing trend was observed in the masseter muscle activity in the TMD group, relative to that in the healthy group. However, the findings of our study could not determine whether the increased muscle activity is caused by TMD pain or contributes to the onset of myalgia. Increased masseter muscle activity could be associated with not only the development of TMD but also elevated pain sensitivity of the muscles and increased incidence of migraine in patients with TMD (28). Yen et al. (29) reported that there were no significant differences between the bilateral sEMG of the masseter and temporalis muscles in healthy adults, and the electrical activity of the temporalis muscles was higher than that of the masseter muscles during habitual postures (30). As mastication is a coordinated neuromuscular function involving the masseter muscle function and mandible movements, treatment strategies for patients with TMD should target modification of the masseter muscle activity.
The relationship between mandibular position and head posture has been documented in previous literature (31). The mandible and associated musculature are essential parts in the functioning of the stomatognathic system, which involves various structures including the TMJ, the depressor and elevator muscle of the mandible, and the oral structures. EMG signals reflect the electrical activity and the function of these muscles, aiding in the development of specific treatment programs for TMD patients according to different facial characteristics. A thorough understanding of the mechanisms behind the main characteristics of the different head positions is important for physical therapists and patients to correct FHP and maintain a relaxed position. However, certain limitations to the study should be considered. First, this was a cross-sectional study indicating that the head and neck posture was associated with the function of the involved muscles. Second, although the study found that electrical activity of the muscles in TMD patients was altered relative to that in healthy subjects, it was not sufficient to conclude that these changes could contribute to the development of TMD. Third, sEMG has certain limitations on accuracy of measuring muscle fatigue and strain compared to needle EMG, and it is necessary to be careful when interpreting the research results. Further study should investigate the relationship between muscle function and the development of TMD.
In conclusion, we found greater electrical activity of the bilateral masseter muscles in TMD patients than in the healthy group in the relaxation position, NHP, and relaxation position with tooth contact; this seems to be associated with the development of TMD. Additionally, the electrical activity of the bilateral anterior temporalis muscles and masseter muscles was significantly higher in the relaxation position with tooth contact. Therefore, based on the findings of our study, physical therapists should focus patient education on maintaining a relaxed position and develop appropriate rehabilitation programs to reduce muscle activity in TMD patients.
Acknowledgments
Funding: This work was supported by the Shanghai Municipal Science and Technology Major Project (Grant No. 19441908400) and the Construction plan of clinical diagnosis and treatment center in Fengxian District (fxlczlzx-a-201706).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1850
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1850
Peer Review File: Available at http://dx.doi.org/10.21037/apm-20-1850
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1850). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (NO.: 2018-96-T87) and informed consent was taken from all individual participants. This clinical trial has been registered in the Chinese Clinical Trial Registry (www.chictr.org.cn; registration number ChiCTR1800018369).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gonzalez HE, Manns A. Forward head posture: its structural and functional influence on the stomatognathic system, a conceptual study. Cranio 1996;14:71-80. [Crossref] [PubMed]
- Ohmure H, Miyawaki S, Nagata J, et al. Influence of forward head posture on condylar position. J Oral Rehabil 2008;35:795-800. [Crossref] [PubMed]
- Olivo SA, Bravo J, Magee DJ, et al. The association between head and cervical posture and temporomandibular disorders: a systematic review. J Orofac Pain 2006;20:9-23. [PubMed]
- Cortese S, Mondello A, Galarza R, et al. Postural alterations as a risk factor for temporomandibular disorders. Acta Odontol Latinoam 2017;30:57-61. [PubMed]
- Di Giacomo P, Ferrara V, Accivile E, et al. Relationship between Cervical Spine and Skeletal Class II in Subjects with and without Temporomandibular Disorders. Pain Res Manag 2018;2018:4286796 [Crossref] [PubMed]
- Hong SW, Lee JK, Kang JH. Relationship among Cervical Spine Degeneration, Head and Neck postures, and Myofascial Pain in Masticatory and Cervical Muscles in Elderly with Temporomandibular Disorder. Arch Gerontol Geriatr 2019;81:119-28. [Crossref] [PubMed]
- Kang JH. Effects on migraine, neck pain, and head and neck posture, of temporomandibular disorder treatment: Study of a retrospective cohort. Arch Oral Biol 2020;114:104718 [Crossref] [PubMed]
- Tingey EM, Buschang PH, Throckmorton GS. Mandibular rest position: a reliable position influenced by head support and body posture. Am J Orthod Dentofacial Orthop 2001;120:614-22. [Crossref] [PubMed]
- Muñoz-García D, Gil-Martínez A, López-López A, et al. Chronic Neck Pain and Cervico-Craniofacial Pain Patients Express Similar Levels of Neck Pain-Related Disability, Pain Catastrophizing, and Cervical Range of Motion. Pain Res Treat 2016;2016:7296032 [Crossref] [PubMed]
- López-de-Uralde-Villanueva I, Beltran-Alacreu H, Paris-Alemany A, et al. Relationships between craniocervical posture and pain-related disability in patients with cervico-craniofacial pain. J Pain Res 2015;8:449-58. [PubMed]
- Armijo-Olivo S, Magee D. Cervical musculoskeletal impairments and temporomandibular disorders. J Oral Maxillofac Res 2013;3:e4 [PubMed]
- von Piekartz H, Pudelko A, Danzeisen M, et al. Do subjects with acute/subacute temporomandibular disorder have associated cervical impairments: A cross-sectional study. Man Ther 2016;26:208-15. [Crossref] [PubMed]
- Uritani D, Kawakami T, Inoue T, et al. Characteristics of upper quadrant posture of young women with temporomandibular disorders. J Phys Ther Sci 2014;26:1469-72. [Crossref] [PubMed]
- Rocha T, Castro M, Guarda-Nardini L, et al. Subjects with temporomandibular joint disc displacement do not feature any peculiar changes in body posture. J Oral Rehabil 2017;44:81-8. [Crossref] [PubMed]
- Šidlauskienė M, Smailienė D, Lopatienė K, et al. Relationships between Malocclusion, Body Posture, and Nasopharyngeal Pathology in Pre-Orthodontic Children. Med Sci Monit 2015;21:1765-73. [Crossref] [PubMed]
- Yilmaz G, Ugincius P, Sebik O, et al. Tonic activity of the human temporalis muscle at mandibular rest position. Arch Oral Biol 2015;60:1645-9. [Crossref] [PubMed]
- Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J Oral Facial Pain Headache 2014;28:6-27. [Crossref] [PubMed]
- Joy TE, Tanuja S, Pillai RR, et al. Assessment of craniocervical posture in TMJ disorders using lateral radiographic view: A cross-sectional study. Cranio 2019; Epub ahead of print. [Crossref] [PubMed]
- Cheung Lau HM, Wing Chiu TT, Lam TH. Clinical measurement of craniovertebral angle by electronic head posture instrument: a test of reliability and validity. Man Ther 2009;14:363-8. [Crossref] [PubMed]
- Hermens HJ, Freriks B, Disselhorst-Klug C, et al. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 2000;10:361-74. [Crossref] [PubMed]
- Gadotti I, Hicks K, Koscs E, et al. Electromyography of the masticatory muscles during chewing in different head and neck postures - A pilot study. J Oral Biol Craniofac Res 2020;10:23-7. [Crossref] [PubMed]
- Piancino MG, Dalmasso P, Borello F, et al. Thoracic-lumbar-sacral spine sagittal alignment and cranio-mandibular morphology in adolescents. J Electromyogr Kinesiol 2019;48:169-75. [Crossref] [PubMed]
- Baba K, Haketa T, Sasaki Y, et al. Association between masseter muscle activity levels recorded during sleep and signs and symptoms of temporomandibular disorders in healthy young adults. J Orofac Pain 2005;19:226-31. [PubMed]
- Funato M, Ono Y, Baba K, et al. Evaluation of the non-functional tooth contact in patients with temporomandibular disorders by using newly developed electronic system. J Oral Rehabil 2014;41:170-6. [Crossref] [PubMed]
- van der Meulen MJ, Ohrbach R, Aartman IH, et al. TMD patients' illness beliefs and self-efficacy related to bruxism. J Orofac Pain 2010;24:367-72. [PubMed]
- Bedi S, Sharma A. Management of temporomandibular disorder associated with bruxism. J Indian Soc Pedod Prev Dent 2009;27:253-5. [Crossref] [PubMed]
- Tecco S, Tetè S, Festa F. Electromyographic evaluation of masticatory, neck, and trunk muscle activity in patients with posterior crossbites. Eur J Orthod 2010;32:747-52. [Crossref] [PubMed]
- Sandoval C, Díaz A, Manríquez G. Relationship between craniocervical posture and skeletal class: A statistical multivariate approach for studying Class II and Class III malocclusions. Cranio 2021;39:133-40. [Crossref] [PubMed]
- Yen CI, Mao SH, Chen CH, et al. The correlation between surface electromyography and bite force of mastication muscles in Asian young adults. Ann Plast Surg 2015;74:S168-72. [Crossref] [PubMed]
- Melo DG, Bianchini EM. Relationship between electrical activity of the temporal and masseter muscles, bite force, and morphological facial index. CoDAS 2016;28:409-16. [Crossref] [PubMed]
- Woda A, Pionchon P, Palla S, et al. Regulation of mandibular postures: mechanisms and clinical implications. Crit Rev Oral Biol Med 2001;12:166-78. Erratum in: Crit Rev Oral Biol Med 2003;14:317. [Crossref] [PubMed]


