
Management of nonbacterial thrombotic endocarditis (NBTE) in advanced non-small cell lung cancer (NSCLC) patients with driver mutation: two case reports
Introduction
NBTE is a rare disease that most often found post mortem with a rate of 0.9% to 1.6% in autopsy series (1-6). It refers to the development of sterile vegetations of fibrin and platelets on the cardiac valves without systemic bacterial infection. NBTE was reported in different age groups, and most commonly in patients aged 40–80 with no sex predilection (7-9). Malignant neoplasms, particularly adenocarcinomas, are the common underlying diseases associated with NBTE. In recent years, significant advances in targeted therapy have been made, but the effectiveness in treating NBTE in patients with severe lung cancer is poorly reported. We describe two cases of severe NBTE in patients with stage IV lung adenocarcinoma harboring driver gene mutations, one with EGFR mutation, the other with ALK fusion oncogene. Both patients were treated with targeted and anticoagulation therapies, with significant improvement. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-251).
Case presentation
Case 1
A 59-year-old man who presented with 3 days of severe dyspnea and chest pain was transferred to The First Affiliated Hospital of Guangzhou Medical University for further treatment in July 2014. His Eastern Cooperative Oncology Group Performance Status (ECOG PS) score was 4 points. Two months prior, an echocardiogram revealed pericardial effusion for which he underwent pericardiocentesis. Adenocarcinoma cells were found in the pericardial effusion, leading to a diagnosis of right lung adenocarcinoma with intrapulmonary and multiple bone metastases based on the findings of positron emission tomography combined with computed tomography (PET-CT). He was treated with traditional Chinese medicine prior to the current hospital presentation.
Initial laboratory findings of the current admission were notable for leukocytosis, high levels of D-dimer and B-type natriuretic peptide (BNP) (white blood cells 23.56×109/L, neutrophil ratio 86.9%, platelets 135×109/L, hemoglobin (Hb) 127 g/L, pH 7.479, carbon dioxide partial pressure 24.4 mmHg, oxygen partial pressure 48.4 mmHg, bicarbonate concentration 17.9 mmol/L, high-sensitivity CRP (hsCRP) 159.87 mg/L, D-dimer 5,073 ng/mL, BNP 4,028 pg/mL, albumin 20.7 g/L, potassium 3.19 mmol/L, sodium 167 mmol/L, chlorine 133 mmol/L). His chest X-ray revealed right lung cancer with metastasis to both lungs and cancerous lymphangitis, anterior pulmonary bullae of the left upper lung, a small amount of pleural effusion on the left lung, and increased heart shadow. His cardiac color-Doppler ultrasound and heart function test indicated widening of the aorta, a mobile mass (Figure 1) measuring 22×21 mm attached to the anterior wall of the ascending aorta, which required further differentiation between cancer-associated embolus and thrombus, no abnormality in left ventricular systolic function, but decreased diastolic function, and large pericardial effusion (Figure 1).
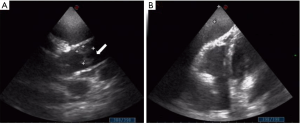
Notably, on the first day of the current admission, he suddenly developed shortness of breath and chest tightness. He was unable to lie down, his ejection fraction (EF) decreased from 73% to 51%, and he underwent a second emergency pericardiocentesis after color Doppler revealed pericardial effusion. The following therapy was started immediately: imipenem and vancomycin for potential infection, and low-molecular-weight heparin for anticoagulation. In addition, nutritional support, regulation of blood sugar, correction of electrolyte disorders and other treatments were given. His blood cultures taken on the first day of the current admission revealed no growth of bacteria 2 days later. In combination, the clinical and imaging findings were suspicious for cancer-associated NBTE.
We recommended the patient request his prior paraffin specimens from his local hospital for us to evaluate whether a genetic test could be performed in the hope of instituting targeted therapy. On day 6 of hospitalization, he was diagnosed with lung adenocarcinoma with EGFR 19 exon deletion mutation. After a 9-day administration of erlotinib 150 mg qd, the vegetations disappeared on echocardiographic examination, the platelet count returned to normal, and the pericardial and pleural effusions were significantly reduced. On day 9 of hospitalization, the antibacterial therapy was switched from imipenem plus vancomycin to piperacillin plus tazobactam due to his improved symptoms. On day 13, routine blood testing revealed white blood cells 8.9×109/L, neutrophil ratio 86.8%, Hb 95 g/L, platelets 86×109/L, potassium 2.75 mmol/L, sodium 152.1 mmol/L, and chlorine 113.8 mmol/L. Liver function test results were: alanine aminotransferase 79 U/L, total protein 52.6 g/L, albumin 30.3 g/L, γ-glutamyl transpeptidase 468.7U/L, hsCRP 88.04 mg/L, BNP precursor 5,434 pg/mL and D-dimer 2,806 ng/mL. Significant improvement was observed on repeat chest X-ray (Figure 2). His ECOG PS score was 3 points at discharge. Over the next 2 months later, the D-dimer level gradually returned to normal, and the echocardiographic EF remained ≈58%. The patient survived for at least another year based on his hospital record and did not show symptoms of deep venous thrombosis (DVT) or recurrence of vegetations in the heart by ultrasound.
Case 2
A 55-year-old woman who had been diagnosed 1 year prior as having lung cancer was transferred to The First Affiliated Hospital of Guangzhou Medical University for further treatment in March 2015. Enhanced CT showed lung cancer in the basal segment of the right lower lobe with right hilar and multiple mediastinum lymph node metastases, with a shadow of a mass in the right adrenal gland, suggesting metastasis. PET-CT revealed mildly active focal bone metabolism in the 4th left anterior rib and the 3rd lumbar spinous process, suggesting benign lesions. Bronchofiberoscopy and pathological biopsy performed 3 days later indicated non-small cell lung cancer (NSCLC) with characteristics of squamous cell carcinoma and adenocarcinoma, and ALK fusion gene positive. Chemotherapy with paclitaxel liposome and etoposide combined with cisplatin was started on day 8 of hospitalization. However, her disease progressed and she showed neurological symptoms consistent with a brain metastasis. On day 26, considering the limited effect of chemotherapy and the patient being ALK positive, she was started on crizotinib 250 mg bid and temozolomide 100 mg qd for the brain metastasis. In addition, she had eight sessions of cranial radiation therapy in August–September 2015. In late March 2016, the patient presented with pain and swelling of the left lower extremity and was diagnosed with DVT. The next day, she was given the first cycle of gemeitabine, and 8 days later placement of a vena cava filter in the left lower extremity was performed and she was administered rivaroxaban for anticoagulation. Her chemotherapy and crizotinib were suspended. Between May and June 2016, the patient received three courses of gemeitabine. After her last course of gemeitabine, her blood platelets and Hb had decreased (lowest Hb was 61 g/L, lowest platelet was 8×109/L) for which she received erythrocyte and platelet transfusions.
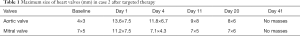
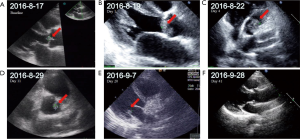
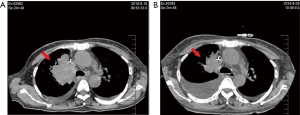
The patient was re-admitted in August 2016 because she presented with chest tightness, palpitations and asymmetric edema of both lower limbs. Her ECOG PS was 4 points. Her platelet count had declined to 8×109/L with D-dimer of 14,647 ng/mL. Color-Doppler ultrasound showed multiple thrombi on aortic and mitral valves, the largest measuring 13.6×7.5 mm. Her EF had decreased to 58% and BNP was 14,647.00 pg/mL. Emergency consultation with cardiologists raised suspicion of infective endocarditis and atrial fibrillation. She was given vancomycin and meropenem as empirical treatment but her condition did not improve. Without notable signs of infection, she was suspected to have cancer-associated NBTE and an 11-day treatment with ceritinib was begun. Some of the thrombi in color-Doppler ultrasound shrank or disappeared (Table 1, Figure 3). Her symptoms improved greatly and CT revealed that the tumor was smaller after targeted therapy (Figure 4) Her D-dimer level decreased to 6,599 ng/mL and Her ECOG PS SCORE was 3 points when discharged. She was advised to continue the targeted therapy and anticoagulants. After 1 month, her D-dimer decreased to 4,181 ng/mL, all the thrombi on the heart valves disappeared on color-Doppler ultrasound, and her EF recovered to 65%. Written informed consent was given by the patients for publication of this case report and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Full table
Discussion
NBTE is not common and it is the development of noninfectious lesions of heart valves, mostly found in patients with advanced malignancy. NBTE is often an autopsy finding with only a small amount of antemortem cases. Patients with NBTE show the signs or symptoms of systemic embolization. NBTE can evolve quickly, eventually preventing any chance of treatment targeting the primary cause thus a timely diagnosis and a proper therapy are very important. We presented two cases with the aim of improving its management in advanced NSCLC patients. Here we discuss the current understanding of NBTE, including its epidemiology, pathogenesis, clinical presentation, evaluation, diagnosis, and treatment.
Epidemiology
It is reported that, patients with malignancy have a higher rate of NBTE (1.25% vs. 0.2%) compared with the general population, in autopsy series (2,3). Comparisons among malignancies indicated that adenocarcinoma has higher rates of NBTE (e.g., lung, colon, ovary, biliary and prostate) (3,10). In both of our cases, the patients had late-stage lung adenocarcinoma, indicating a higher risk of NBTE. Therefore, every patient with advanced lung cancer should be tested to rule out the possibility of NBTE or any other thrombi in the body. Once discovered, patients with NBTE with or without evidence of systemic emboli (including central nervous system lesions) are routinely anticoagulated provided there is no contraindication (e.g., central nervous system bleeding). Treatment of the underlying condition, such as malignancy, should be attempted when appropriate to interrupt the progression of cerebral thromboembolism in NBTE.
Pathogenesis
To date, the pathogenesis of NBTE is not complete understood by scientists. The potential reasons are endothelial damage with subsequent exposure of subendothelial tissue to circulating platelets, immune complex deposition, hypoxia with resulting plasma tissue factor abnormalities, and hypercoagulability possibly due to hypo- and hyper-fibrogenemia, thrombocytopenia, decreased factors V, VIII, and XII and antithrombin III, and increased fibrinolysis (11). In our cases, both patients presented with decreased platelet count and elevated D-dimer but the etiology remains unknown.
Cancer cells can promote the activation of blood coagulation directly by generating thrombin, or indirectly by stimulating endothelial cells and circulating mononuclear cells to synthesize and express several procoagulant factors (12). Thromboembolic events are common in cancer, which complicate the cancer management and are associated with increased mortality. Previous studies have reported that lung cancer have a high incidence of venous thromboembolism (VTE), including deep vein thrombosis (DVT), pulmonary embolism, other depositions in the upper extremities or other areas. As for arterial thromboembolic events, vegetations in the heart such as NBTE or stroke can be seen in NSCLC.
Clinical presentation
NBTE is often an autopsy finding (2) because patients are typically asymptomatic until embolization occurs. Thus physicians should pay attention to the signs and symptoms of embolization on patients with advanced lung cancer. The patient in case 2, who had a history of DVT after diagnosed with lung cancer, should have been considered as high-risk for NBTE. Both patients, by having atypical findings, challenged the differential diagnosis between bacterial and sterile valvular heart disease. The vegetations in NBTE are easily dislodged in comparison with that in infective endocarditis, which lead to poor outcomes such as the most notably recurrent or multiple ischemic cerebrovascular strokes that cause substantial morbidity in patients with cancer.
When the thrombi deposit in arterial system, the NBTE patients with cancer have a high risk to develop a stroke. There are differences in the causes and mechanisms of stroke due to changes in blood coagulation and/or NBTE. And such differences are various in different situation, which may be related to the cancer and the patient condition per se. NBTE is characterized by the deposition of sterile platelet thrombi on heart valves, mostly on aortic and mitral. The initiating factor in the pathogenesis of NBTE is not clear, but the endothelial injury in the setting of a hypercoagulable state is considered as important for the development of NBTE. Circulating cytokines, such as tumor necrosis factor or interleukin-1, may cause endothelial damage, which may lead to platelet deposition, especially in the presence of an activated coagulation system (e.g., disseminated intravascular coagulation, malignancy, antiphospholipid syndrome) to result in local deposition of platelets as well as inflammatory molecules. Such vegetations are more likely to embolize and cause extensive infarction. Thus, clinicians must look for signs of increased risk for NBTE, which relies on individual risk factors, in cancer-related hypercoagulability, being on anticancer drugs and undergoing radiotherapy etc.
Evaluation and diagnosis
Currently, no specific test can confirm the diagnosis of NBTE. Echocardiography is unable to distinguish vegetations due to thrombus or infection from those due to aggregations of platelets and fibrin as seen in NBTE. In our cases, both patients were initially suspected to have infective endocarditis or NBTE after visualization of vegetations. In case 2, the patient was suspected to have infectious endocarditis when first admitted to hospital with symptoms of heart failure. She was given empirical antibiotics but showed no obvious improvement. A timely evaluation should be made for early diagnosis of cancer-associated NBTE, which is based on full assessment with complete blood count, blood chemistry, workups for hypercoagulable state, negative blood cultures of bacteria and absence of prior history of heart disease or rheumatic disease. In our cases, the relatively promising outcomes after targeted therapy also helped to support the diagnosis of cancer-related NBTE. NBTE can evolve quickly, eventually preventing any chance of treatment targeting the primary cause. In the management of patients with severe NSCLC, color-Doppler ultrasound of the heart and lower extremities is recommended as routine for early detection of such cancer-related complications.
Treatment
Here are the components in the treatment of NBTE: (I) systemic anticoagulation is necessary to limit the recurrent embolization. (II) Treatment aiming at the underlying disease should be started if possible. (III) Surgical intervention on the affected cardiac valve could be considered. For patients with NBTE, surgery should be considered as an individualized option according to their performance status and life expectancy. In patients with advanced lung cancer combined with NBTE, due to the poorer condition caused by cancer, surgery is usually not a choice. Thus, treatment options are limited for advanced cancer patients with NBTE, who have a poor prognosis.
Fortunately, the past decades have witnessed the dramatic shift in treatment of lung cancer. The oncogenic driver mutations identified in NSCLC with the increasing number of clinically available signal transduction pathway inhibitors targeting these driver mutations, offers a tremendous opportunity to enhance the outcomes of patients. As we understand more of the molecular pathways that drive malignancy in NSCLC and other neoplasms, agents that target specific molecular pathways in malignant cells develop rapidly. Such agents can preferentially kill malignant cells, but relatively innocuous to normal cells.
In the absence of contraindications, treatment for NBTE combined with lung cancer, systemic anticoagulation and targeted therapies can be started whenever possible, to ameliorate symptoms, control the underlying malignancy, and to prevent further thromboembolic episodes. In our cases, after targeted therapy, the vegetations in heart of both patients vanished, the platelet count recovered and ECOG PS score changed from 4 to 3.
In previous studies, various molecular abnormalities have been discovered which affect the characterization of particular subsets of patients with NSCLC. In the era of precision medicine, such subclassification is critical for personalizing patient care and defining patient categories with differing prognoses. Specific targeted therapies are widely used for patients with advanced disease with specific molecular features. Activating mutations in EGFR define a subset of patients with adenocarcinoma that more frequently found in patients who are never smokers, women, Asian. EGFR tyrosine kinase inhibitors (osimertinib, erlotinib, gefitinib, afatinib) show great efficacy in these patients. And such patients have a better prognosis than those without EGFR mutations.
The presence of the ROS1 or EML4-ALK fusion oncogene defines other NSCLC subsets that are more frequent in nonsmokers or former smokers and occurs at a younger age. Other less frequent driver mutations in non-small cell lung cancer were identified, including human epidermal growth factor receptor 2 (HER2), BRAF, neurotrophic receptor tyrosine kinase (NTRK), RET and MET. There are corresponding targeted therapies for certain genotypes. If no mentioned mutation, expression of tumor PD-L1 can be used to predict the response to immunotherapies and to guide the strategy in the first-line or subsequent-line treatment settings.
There are concerns on the waiting period for genetic tests would affect the prognosis chemotherapy-treated de novo Metastatic NSCLC patients without a driver mutation. It only takes 1–2 days to identify EGFR mutation in patients with lung cancer by PCR, 2 days for ALK fusion oncogene by immunohistochemistry in our hospital. Such a short waiting period is worthwhile because of the necessity of identifying the subset of the lung cancer. Treatment for lung cancer depends on the assessment of the patient's overall medical condition before the genetic test result coming out. If the patients diagnosed with lung cancer are in an urgent scenario without genetic tests results facilitating us to make clinical decision, chemotherapy might be in our consideration. Thus, if patients were chemotherapy-treated de novo metastatic NSCLC patients without a driver mutation, the waiting period for genetic tests would not affect their prognosis.
These are two rare reported cases of cancer-associated NBTE in advanced NSCLC. Both patients shared similarities in clinical manifestation, treatments and outcomes. They were in their late 50 s and had severe stage IV lung adenocarcinoma (ECOG PS score: 4) before treatment. Both were discharged in stable condition with plans for outpatient therapy. No recurrences of NBTE were reported before their deaths.
Our report highlights the necessity of timely diagnosis and gene classification for NSCLC patients (ECOG PS score ≥3), the importance of concurrent treatment for the cancer and its complications, and further stresses the effectiveness of targeted therapy in the treatment of NBTE in patients with advanced lung cancer harboring driver gene mutations. The rapid pathological diagnosis and genetic classification were crucial in these two successful rescues, combining targeted therapy with anticoagulation therapy. However, it should be noted that the follow-up schedules of patients were not clearly known, and we did not confirm the diagnosis with biopsy.
In summary, the lessons we learned from these two cases are: (I) to treat NBTE in patients with advanced NSCLC with driver mutations, targeted therapy should be instituted with anticoagulation as an effective strategy, which requires rapid pathological diagnosis and genetic classification; (II) color-Doppler ultrasound can be used as a routine screening for NBTE in NSCLC patients; and (III) a history of DVT, PTE or other thrombotic events indicates potential for NBTE or indirect evidence of the progression of cancer. To enhance the outcome of advanced cancer patients, a large and long-term study is required to provide a comprehensive guideline for the management of NBTE.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-251
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-251). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was also given by the patients for publication of this case report and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deppisch LM, Fayemi AO. Non-bacterial thrombotic endocarditis: clinicopathologic correlations. Am Heart J 1976;92:723-9. [Crossref] [PubMed]
- Rosen P, Armstrong D. Nonbacterial thrombotic endocarditis in patients with malignant neoplastic diseases. Am J Med 1973;54:23-9. [Crossref] [PubMed]
- González Quintela A, Candela MJ, Vidal C, et al. Non-bacterial thrombotic endocarditis in cancer patients. Acta Cardiol 1991;46:1-9. [PubMed]
- Steiner I. Nonbacterial thrombotic endocarditis--a study of 171 case reports. Cesk Patol 1993;29:58-60. [PubMed]
- Maté del Tío M, Gómez Cerezo J, Garcés Jiménez MC, et al. Nonbacterial thrombotic endocarditis: a review of a necropsy series. Rev Clin Esp 1997;197:9-13. [PubMed]
- Eiken PW, Edwards WD, Tazelaar HD, et al. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985-2000. Mayo Clin Proc 2001;76:1204-12. [Crossref] [PubMed]
- el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 2007;12:518-23. [Crossref] [PubMed]
- Mazokopakis EE, Syros PK, Starakis IK. Nonbacterial thrombotic endocarditis (marantic endocarditis) in cancer patients. Cardiovasc Hematol Disord Drug Targets 2010;10:84-6. [Crossref] [PubMed]
- Lopez JA, Ross RS, Fishbein MC, et al. Nonbacterial thrombotic endocarditis: a review. Am Heart J 1987;113:773-84. [Crossref] [PubMed]
- Borowski A, Ghodsizad A, Cohnen M, et al. Recurrent embolism in the course of marantic endocarditis. Ann Thorac Surg 2005;79:2145-7. [Crossref] [PubMed]
- Asopa S, Patel A, Khan OA, et al. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg 2007;32:696-701. [Crossref] [PubMed]
- Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 2012;366:601-9. [Crossref] [PubMed]
(English Language Editor: K. Brown)



