
Research progress of metformin in gestational diabetes mellitus: a narrative review
Introduction
Gestational diabetes mellitus (GDM) refers to different degrees of abnormal glucose metabolism during pregnancy, where blood glucose levels do not reach the level of overt diabetes, accounting for 80–90% of pregnancy with hyperglycemia (1). With lifestyle changes, increased pregnancy age, and increased body mass index (BMI) in recent years, GDM incidence has increased to approximately 15–20% (2).
Compared with healthy pregnant women, pregnant women with GDM are more likely to have maternal and infant complications, and tend to have type 2 diabetes (3), cardiovascular diseases, dyslipidemia and metabolic disorders after delivery (4-6). Therefore, active treatment significantly impacts maternal and infant prognosis. Blood glucose control during pregnancy depends mostly on a combination of health interventions, medications and blood glucose self-monitoring (7). At present, some institutions suggest blood glucose management through dietary therapy (8-12) and that insulin or oral hypoglycemic drugs should be used in patients who are unable to control blood glucose effectively through diet and exercise alone. Insulin has always been the preferred hypoglycemic drug for women with GDM who cannot control their blood sugar through lifestyle interventions; however, insulin treatment has some drawbacks, including multiple daily injections, the possibility of hypoglycemia, increased appetite and weight gain (13).
Metformin is a drug that plays a role in inhibiting liver gluconeogenesis and increasing peripheral insulin sensitivity. A large randomized controlled trial (RCT) published in 2008 showed that metformin alone or insulin used in combination with metformin are safe and effective treatment options for women with GDM (14). Currently, the largest and longest clinical trial of metformin in diabetes prevention is the Diabetes Prevention Plan/Diabetes Prevention Plan Outcome Study (DPP/DPPOS) (15). This RCT—based on data from 3,234 adults—also showed that metformin might help prevent diabetes at long-term follow-up; however, the use of metformin is still controversial. Some studies support the use of metformin for glycemic control in pregnant women (13,16), while others show an increased risk of adverse events (17).
The presence of polycystic ovary syndrome (PCOS)—one of the most common endocrine diseases related to hyperinsulinemia and insulin resistance in women of childbearing age—increases the risk of developing GDM (18). One study found that adherence to metformin in patients with PCOS reduced the risk of GDM and gestational hypertension syndrome (19) as well as the rate of miscarriage in early pregnancy (20); however, according to the 2020 American Diabetes Association (ADA) guidelines, metformin should only be used in the first trimester of pregnancy to treat PCOS and to induce ovulation. At present, GDM has not been included as an indication for metformin use in China. Therefore, to further understand the safety and efficacy of metformin in the treatment of GDM, we conducted an up-to-date literature review of studies investigating the use of metformin in the treatment of GDM.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/apm-21-192).
Safety of metformin for offspring in pregnant women with GDM
Concerns around the safety of metformin during pregnancy has partly pertained to potential drug movement across the placental barrier. Eyal et al. (21). found that metformin concentration in fetal cord blood could reach levels as high as the maternal level. The safety of metformin for offspring of pregnant women with GDM is discussed.
Effects of prenatal exposure to metformin on the long-term growth and development of offspring
One study (the MIG experiment: the Metformin in Gestational Diabetes RCT) found that at 2 years of age, the offspring of mothers who took metformin during pregnancy had a higher subcutaneous fat content than those of mothers who took insulin, but had similar overall fat content (22). The study suggests that exposure to metformin in utero may affect the fat deposition patterns of offspring leading to a healthier growth pattern, with more subcutaneous fat reserves and less ectopic or visceral fat. But the study also concluded that follow-up at a later stage is important, and that postpartum effects on growth may outweigh the effects of late trimester exposure to metformin (22). At 9 years of age, it was found that the children exposed to metformin were larger in weight, arm, waist circumference, waist-height ratio, BMI and abdominal fat mass (23). Further analyzing the causes, the study suggests that in addition to the role of metformin, fetal nutritional supply, gender, and the post-birth environment may be important additional factors to consider. There are important interactions between these factors and metformin, which may affect pregnancy and long-term prognosis (23). Carlsen et al. found that children (regardless of the gender) born to mothers who received metformin treatment during pregnancy were heavier at 1 year of age (24). This weight difference persisted after adjusting for factors affecting weight development such as gestational age, birth weight, maternal BMI, maternal height, and duration of breastfeeding, and could not be attributed to the “big-mom-big-baby” phenomenon. Similarly, Ijäs et al. found that children exposed to metformin were heavier at 12 months, and taller and heavier at 18 months (25). To further understand the long-term effects of prenatal metformin exposure on the offspring health, a systematic review and meta-analysis were performed using published RCTs (26). The review included 11 RCTs—with a total of 823 GDM or PCOS mothers—and showed that prenatal exposure to metformin was associated with greater offspring weight gain, while parameters of body composition, metabolism and neurophysiological development were similar between the metformin and insulin groups. Therefore, according to the current study, it has been found that the offspring of GDM pregnant women treated with metformin are more likely to experience weight gain. However, the importance of environmental factors in offspring growth and metabolism should not be overlooked. Therefore, further studies are needed to investigate the effects of fetal exposure to metformin on the body composition of the offspring.
Effects of prenatal exposure to metformin on offspring abnormalities
Research progress in animal experiments
The relationship between prenatal metformin exposure and incidence of offspring abnormalities remains equivocal in animal studies. The main mechanism of action of metformin is by increasing the activation of AMP-activated protein kinase (AMPK) (27). AMPK activation may interfere with the expression of embryonic genes, in particular inhibiting the expression of PAX3—a gene associated with neural tube closure—which consequently increases risk of neural tube defects (28). Denno and Sadler have demonstrated that metformin can lead to decreased protein in the yolk sac and delayed closure of the neural tube in animals (29). Meanwhile, metformin has been shown to have anti-folic acid effects similar to some chemotherapy drugs (28), suggesting that metformin has some teratogenic effects; however, Lee et al. found that although metformin increased AMPK and inhibited PAX3 expression in mouse embryonic stem cells in vitro, no negative outcome was observed, indicating that metformin does not cause deformity in mice (30).
Research progress in clinical application
Clinical studies suggest that metformin has little effect on embryos (31). Metformin has very limited transport via the organic cationic transporter (OCT), so the possibility of exposing embryonic tissue to metformin is extremely low. Conversely, certain physiological and metabolic characteristics of embryos may limit the teratogenic effect of metformin. At present, metformin has shown no significant effect in clinical practice on the occurrence of offspring abnormalities in pregnant women with GDM. A meta-analysis conducted by Gilbert and Koren involving eight metformin studies found that exposure to metformin in the first trimester did not increase the risk of fetal malformation (32). The study found that the mean malformation rate in the untreated group was approximately 7.2%, the rate found in the metformin group was 1.7%. After adjustment for publication bias, metformin treatment in the first trimester was associated with a statistically significant 57% protective effect (32). Bolton et al. conducted a RCT in which 66 women with PCOS in the experimental group received metformin in the first trimester of pregnancy, while 66 women in the control group underwent metformin withdrawal in the first trimester of pregnancy (all women were previously on metformin for their PCOS prior to pregnancy); no significant differences in congenital malformations were found in the metformin group compared to the control group (33). In 2019, Bao et al. conducted a meta-analysis, which also found that maternal use of metformin during the first trimester of pregnancy did not increase the risk of congenital malformations in offspring (34). From the present study, we found that maternal metformin treatment did not increase the incidence of fetal deformities. However, whether metformin plays a potential role in the prevention and treatment of fetal malformations has not yet been concluded, and more samples are needed for further study.
Efficacy evaluation of metformin in the treatment of GDM
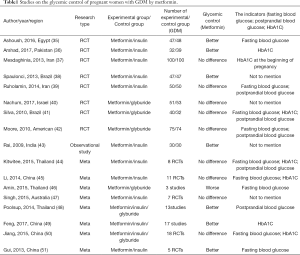
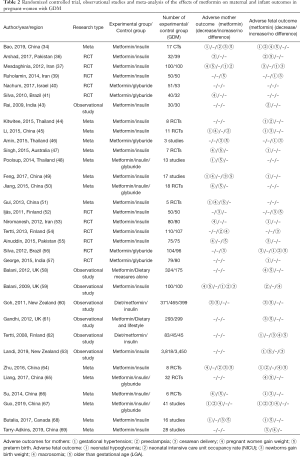
At present, there is a lack of consensus on the efficacy of metformin in the treatment of GDM. Some studies believe that blood glucose levels in women with GDM can be effectively managed with metformin without the occurrence of adverse maternal and infant outcomes. On the contrary, other studies believe that metformin did not have a clear effect on blood glucose control and reduction of adverse maternal and infant outcomes, but instead increased some of the adverse reactions. In the past decade, a number of retrospective and prospective randomized studies, along with related meta-analyses, have been published to compare prenatal blood glucose levels and maternal outcomes in women with GDM managed with different treatments. Therefore, to further understand the therapeutic effect of metformin on GDM, we summarized some studies on the control of blood glucose by metformin in pregnant women with GDM to further explore the effect of metformin on blood glucose in GDM in Table 1 (35-51). At the same time, we also summarized the randomized controlled trials (36-37,39-41,52-57), observational studies (43,58-63) and meta-analyses (34,44-51,64-69) of the effects of metformin on maternal and infant outcomes of pregnant women with GDM, and discussed the effects of metformin on maternal and infant outcomes in GDM in Table 2.
Full table
Full table
Influence on blood glucose of pregnant women
According to Table 1, there are still some differences in the efficacy of metformin in blood glucose control in women with GDM. An RCT by Silva et al. compared the glycemic control of metformin and glyburide in pregnant women with GDM, and found no significant difference in glycemic control (41,42). Ruholamin et al. also found that metformin showed no difference in blood glucose control in pregnant women with GDM compared to insulin in RCTs (39); however, one study found that metformin was more effective than insulin in reducing fasting glucose and glycosylated hemoglobin (70). In view of the existing differences, many researchers have carried out meta-analyses to further explore the effect of metformin on blood glucose in pregnant women. Jiang et al. (50) systematically evaluated the therapeutic effects of metformin, insulin and glyburide in pregnant women with GDM using data from 18 RCTs, and found that there were no differences in the effects of the three drugs on fasting blood glucose and glycated hemoglobin levels; however, a meta-analysis involving 12 RCTs and 5 observational or retrospective case-control studies found that pregnant women in treated with metformin had more stable glycated hemoglobin levels compared with those treated with insulin (49). A meta-analysis by Gui et al. found that the postprandial blood glucose levels in women with metformin-treated GDM were lower than that of those treated with insulin during the first week after treatment (51). Due to different observational indicators adopted by different studies, different time to evaluate blood glucose in pregnant women, and the fact that some GDM patients taking metformin require additional insulin to control blood glucose, which makes it impossible to determine whether metformin has an independent glycemic control effect in all patients. More studies are needed to further evaluate the glycemic control of metformin in pregnant women with GDM.
Influence on maternal outcome
Hypertensive disorders during pregnancy
Table 2 shows that almost all meta-analyses suggested that metformin could reduce the risk of hypertension during pregnancy, despite differing opinions on its effect on development of preeclampsia. One meta-analysis of 11 RCTs—including 2,712 pregnant women with GDM—compared the efficacy of metformin and insulin, and found that metformin was associated with a lower incidence of gestational hypertension, but no significant difference in the incidence of preeclampsia (45). Tertti et al. (54) also showed that there was no statistically significant difference in the incidence of preeclampsia between metformin and insulin groups, although suggested that an increased sample size might have produced significant differences. Guo et al. (67). found that metformin reduced the incidence of preeclampsia compared with insulin on meta-analysis. This is inconsistent with the results of some previous meta-analyses (34,64). Analysis of the cause of the results, found that the sample size in this study conducted by Guo et al. is very rich. Among the 41 studies, a total of 14 studies involving 3402 patients with GDM took preeclampsia as the result of metformin and insulin. The sample size of the studies was significantly larger than that of other meta-analyses, which may be one of the reasons for the statistical difference between metformin and insulin in the incidence of preeclampsia.
Cesarean delivery
Table 2 shows that metformin did not increase the incidence of cesarean section in pregnant women with GDM in most studies. By comparing the metformin group and the insulin group with the diet group, Silva et al. (71). found that the mode of delivery was not significantly different, suggesting that treatment regimen did not affect the mode of delivery. A prospective study comparing women with GDM managed with metformin and insulin with women managed with dietary interventions found that women managed with insulin had a higher rate of cesarean section than women managed with metformin or with dietary interventions (60); however, Ijäs et al. suggested that GDM patients treated with metformin had a higher rate of cesarean section (52).
Pregnant women gain weight
Zhang et al. (72). found that women with GDM treated with metformin had significantly lower BMI, body mass during pregnancy, and overall quality of delivery compared to those treated with insulin.
In a RCT comparing the efficacy of metformin-alone, metformin-combined-with-insulin and insulin-alone groups of women with GDM, Ainuddin et al. found that the insulin-alone group had significantly higher pregnancy weight than those in the metformin-combined-with-insulin group (55). Another meta-analysis showed that pregnant women treated with metformin had less weight gain from registration to 36–37 weeks gestation than those in the insulin group, suggesting metformin to be beneficial in preventing weigh gain, even where metformin was insufficient and required insulin supplementation (49). However, other studies have shown that no statistically significant difference in weight gain between metformin and insulin groups (54).
Preterm birth
Table 2 shows that the relationship between metformin management of GDM and increased risk of premature delivery is inconclusive and controversial. Ainuddin et al. (55) compared metformin-alone, metformin-combined-with-insulin and insulin-alone groups of women with GDM and showed no significant differences between groups for preterm birth rates. Bao et al. (34) also showed that metformin and insulin use in GDM had no significant difference in preterm birth rates. These findings were in contrast to Mesdaghinia et al. whose RCT found that metformin was associated with a lower risk of preterm birth in pregnant women compared to insulin (37); observational studies also support this conclusion (60). Conversely, one meta-analysis suggested that metformin may increase the incidence of premature delivery in pregnant women to some degree (47).
Serum levels of cystatin C (CysC) and homocysteine (Hcy) in pregnant women
Studies have shown that increased Hcy level can reduce insulin sensitivity and increase blood glucose level, which are directly related to the development of disease and maternal and infant complications; however, excessive urination caused by hyperglycemia in GDM patients will lead to excessive loss of water-soluble B vitamins and folic acid and an increase in Hcy serum levels. In GDM, serum CysC can promote the inflammatory response, damage blood vessels, and inhibit the activity of Hcy decomposition enzyme, leading to a vicious cycle of elevated blood glucose. It is therefore often used as a prognostic indicator for GDM and maternal and infant outcomes. One study found that serum CysC and Hcy levels in pregnant women treated with metformin were significantly lower than those treated with insulin (73). Wu et al. also found that metformin combined with insulin is more likely to reduce serum CysC and Hcy concentrations than insulin alone in the treatment of GDM (74-76). To some extent, CysC and Hcy can reflect renal function damage and vascular intima; however, due to the lack of relevant data, further studies are needed to show whether metformin can effectively reduce renal and vascular intima damage in pregnant women with GDM.
Effects on neonatal outcomes
Neonatal hypoglycemia
A recent retrospective cohort study (63) found that metformin significantly reduced the incidence of neonatal hypoglycemia in pregnant women with GDM compared with insulin treatment. One RCT found that neonatal hypoglycemia was higher in the insulin group than in the metformin group (38), while recent meta-analysis suggested that the risk of neonatal hypoglycemia in the metformin group was lower than in the insulin group (34,69). Conversely, another RCT comparing neonatal outcomes in women with GDM treated with metformin versus insulin found no significant differences in the incidence of neonatal hypoglycemia (39). Although there is some disagreement on metformin’s ability to reduce the incidence of neonatal hypoglycemia, we found no reports that suggested metformin increased neonatal hypoglycemia (Table 2).
Neonatal intensive care unit (NICU) occupancy rate
A case-control study compared the pregnancy outcomes of 100 women with GDM taking metformin with those of 100 women with GDM taking insulin and found that the incidence of NICU occupancy was significantly higher in the insulin group (19%) compared with the metformin group (6%) (59). Table 2 shows that multiple meta-analyses have shown that metformin can reduce the rate of neonatal NICU occupancy compared with insulin (34,44-45,67). Additionally, Ainuddin et al. found that the NICU occupancy rate in insulin therapy alone was significantly higher compared with metformin-with-insulin therapy and metformin therapy alone (55); however, other studies have shown no statistically significant difference in NICU occupancy rate and occupancy time between metformin and the insulin management (54).
Birth weight of newborn
Ainuddin et al. (55). compared the neonatal birth weights of insulin therapy, metformin therapy, and metformin-with-insulin therapy groups, and found that birth weights in the insulin therapy group were relatively large, while the differences between the metformin therapy group and the metformin-with-insulin groups were not statistically significant, suggesting that metformin may improve the birth weight. One RCT found that newborn birth weight of the metformin group was lower compared to the insulin group, indicating that metformin could reduce the newborn birth weight to some degree (77). In addition, we found that several meta-analyses showed that metformin reduced birth weights of newborns compared with insulin (45,66,67); however, a recent retrospective cohort study showed no clinically significant difference in neonatal mean birth weight between pregnant women treated with metformin and insulin (63). Another RCT showed no statistically significant difference in the average birth weight between metformin and insulin treatment groups (54).
Macrosomia and large gestational age (LGA)
Macrosomia is one of the most common neonatal complications of GDM. One study suggested that strict control and close monitoring of blood glucose in GDM patients at 32 to 37 weeks of gestation is necessary to reduce the incidence of macrosomia (78). Studies have found that the incidence of macrosomia in metformin-treated women with GDM was significantly lower than that in the insulin-treated group (61). A retrospective cohort study found that metformin-treated women with GDM had significantly lower incidence of macrosomia compared with those treated by diet alone (59). Multiple meta-analyses have also shown that metformin could reduce the incidence of macrosomia and LGA compared with insulin treatment of GDM (44,50,64,65); however, other studies have suggested that metformin use did not significantly reduce incidence of macrosomia and LGA (52,59,62). Ainuddin et al. found that there was no statistically significant difference in the incidence of LGA and macrosomia between metformin therapy, insulin therapy and metformin-with-insulin therapy groups (55). In addition, studies have found that pregnant women treated with metformin have a higher frequency of vitamin B12 deficiency (28). Several studies reported changes in vitamin B12 and folic acid in pregnant women with GDM treated with metformin (70). Reduced levels of folic acid and vitamin B12 may have some effect on the development of mothers and their offspring; however, it appears that these reductions in concentrations may have clinical significance only in women with severe risk deficiency (70).
In addition to the effects of metformin on blood glucose and maternal outcomes in pregnant women with GDM, other adverse reactions may also occur during the course of taking the drug. Gastrointestinal issues—such as nausea, vomiting and diarrhea—are the most common adverse reactions. Such adverse effects decrease over time and may be mitigated by reducing the dose and taking metformin with food (79-80). Recently, it has been reported that a probiotic can improve metformin gastrointestinal tolerance (81). Metformin-associated lactic acidosis (MALA) is the most serious adverse reaction of the medication, but is rare in patients with normal liver and kidney function, with only 3 cases per 100,000 (82). A systematic review showed no difference in the incidence of lactic acidosis between metformin and other oral hypoglycemic agents (83). The presence of lactic acidosis in patients treated with metformin does not mean that it is caused by metformin but may also be due to the presence of other risk factors, such as heart disease, renal insufficiency, chronic lung disease, chronic liver disease, age, and acute gastrointestinal disease. Misbin et al. (84) reported on 66 cases of MALA diagnosis and found that only 47 of them were related to the use of metformin, and of those 47 cases, 43 had other risk factors include heart disease, renal insufficiency, chronic lung disease with hypoxia, and over age 80, with only four of the original cases being truly related to metformin. Others also suggested that the occurrence of MALA was not necessarily caused by the administration of metformin (85-86). The patient's associated complications may have a negative impact on metformin use, especially in chronic kidney disease, which may affect drug excretion. In congestive heart failure and chronic liver disease, this may affect lactic acid clearance and promote lactic acid accumulation. In a retrospective analysis of 559 confirmed cases of metformin associated lactic acidosis (87), Isabelle et al. found that almost all of the reviewed cases (97%) had independent risk factors for lactic acidosis, including: kidney damage, shock, liver damage, dehydration, heart failure, and acute gastrointestinal disease. The study suggested that metformin, when used at therapeutic doses, may not play a role in the development of lactic acidosis or may only play a promoting role, rather than a major role. An increased risk of lactic acidosis related to metformin use is therefore questionable (88). Other less common side effects include altered taste, elevated liver enzymes, and skin erythema or urticaria (82).
Effect of metformin on the incidence of GDM in pregnant women with PCOS
PCOS is the most common endocrine anovulatory infertility disease in women of childbearing age, with a prevalence of around 7–15% (89). Studies have found that PCOS is associated with hyperinsulinemia and insulin resistance which may be further aggravated during pregnancy, making those with this condition prone to GDM (18). A large Swedish cohort-study found that presence of PCOS doubled the risk of developing GDM during pregnancy (90). Begum et al. conducted a RCT with pregnant women with PCOS, where fifty-nine non-diabetic PCOS patients who conceived while taking metformin and different ovulation-inducing agents comprised the sample group. Twenty-nine of them continued metformin throughout pregnancy and 30 did not; in this study, women with PCOS were found to have a nine-fold reduction in GDM risk with metformin treatment (91). Meta-analysis has shown that metformin treatment during pregnancy could reduce the incidence of GDM in pregnant women with PCOS (92); however, another meta-analysis including 11 studies showed that metformin treatment in pregnant women with PCOS had no effect on the incidence of GDM in this population (93). In addition, while meta-analyses have shown that the use of metformin in PCOS could significantly reduce the incidence of abortion and preterm birth, no significant differences were reported for the incidence of GDM, gestational hypertension and preeclampsia with or without metformin treatment (94,95). According to the ADA 2020 guidelines, metformin should be used in the first trimester of pregnancy for the treatment of PCOS and ovulation induction, but that continued use of metformin after the first trimester may increase the risk of adverse pregnancy outcomes in women with PCOS. A prospective, randomized, multicenter trial in pregnant women with PCOS aimed to determine the preventive effects of metformin exposure from first trimester to delivery on pregnancy complications found no significant differences between metformin and placebo in major outcomes related to pregnancy or neonates (96). Tan et al. (94). believed that insulin resistance (IR) and hyperinsulinemia may lead to the tendency of adverse events in women with PCOS. Considering the effect of IR on the physiology of pregnancy, it is suggested that continued treatment with metformin may be beneficial for women with PCOS. However, Gonzalez et al. found that discontinuing metformin after pregnancy diagnosis in PCOS women did not appear to be associated with a higher risk of miscarriage (31). For women with PCOS, prolonged use of metformin after pregnancy diagnosis does not seem to make sense. Currently, studies of the continuation of metformin therapy in the first trimester of pregnancy in PCOS pregnant women have shown that no adverse outcomes appear to occur with continued use of metformin in the first trimester of pregnancy. However, due to limited data, the risks/benefits of continuing metformin therapy in the second and third trimesters of pregnancy for patients with PCOS remain unclear. It is recommended that patients with PCOS should be treated with metformin on a judicious basis, and that clinical guidelines and regulations be carefully followed.
Guidelines and recommendations of metformin for the treatment of GDM and drug regimen
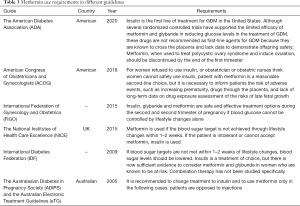
Metformin has not been approved by the Food and Drug Administration (FDA) for the management of GDM and is listed as a Class B drug (70). We found that there is no clear consensus regarding the efficacy of metformin treatment in pregnant women with GDM pertaining to its effects on fetal malformation, long-term growth and development of offspring, blood glucose in pregnant women, and maternal and infant outcomes. We also found that there are significant differences in guidelines regarding metformin treatment for GDM in different countries (Table 3), with some guidelines reporting metformin as a first-line treatment for GDM (9,97) and other guidelines suggesting that there is insufficient evidence for metformin to replace insulin treatment in GDM (8). The use of antidiabetic drugs for blood glucose management during pregnancy is also not recommended in recent Indian guidelines (99), while the latest Australasian Diabetes in Pregnancy Society (ADIPS) (100) and Australian Electronic Treatment Guidelines (eTG) (100) also recommend switching treatment to insulin and using metformin only in patients opposed to insulin injections; this guideline may be related to the lack of long-term safety data for metformin in neonates and fetuses.
Full table
Regarding the dosage of metformin in GDM management, studies have found that, compared with postpartum, the renal clearance rate of metformin in the second and third trimesters was significantly increased (by 29%), in alignment with an increased renal plasma flow and glomerular filtration rate during pregnancy (21,101). Patients may therefore need a higher dose of metformin to achieve a sufficient hypoglycemic effect. The current dose of metformin used to treat women with GDM is 500 to 2,500 mg/day, and the impact of doses over 2,500 mg/day on maternal, fetal, and neonatal safety has not been determined.
Conclusions
Our study found that the offspring of pregnant women with GDM exposed to metformin before birth were more likely to develop a fat metabolism disorder and gain weight during later growth and development. Most studies suggest that metformin will not increase the incidence of offspring malformation in animal experiments or in clinical studies regarding the effect of metformin on offspring deformity incidence.
Although the effect of metformin treatment on various maternal outcomes (gestational hypertension, preeclampsia, cesarean section, weight gain, premature delivery) and fetal outcomes (neonatal hypoglycemia, NICU occupancy, neonatal birth weight gain, macrosomia and LGA) are still divergent, most studies have shown that metformin does not increase the incidence of these adverse outcomes. Therefore, metformin appears to be an effective and safe oral drug to replace insulin in the treatment of GDM. However, due to some study limitations, larger sample sizes are needed to assess the maternal and neonatal complications in GDM treated with metformin and to evaluate long-term follow-up data for children born to women with GDM treated with metformin, to determine the safety of this drug in GDM treatment. In the future, the focus should be on the long-term effects of metformin use during pregnancy on children and their future offspring, which will provide more robust evidence regarding the safety and efficacy of this treatment.
Acknowledgments
We gratefully acknowledge Junwu Wang for providing intellectual support and technical assistance.
Funding: This research was supported by the National Natural Science Foundation of China (Grant No. 81700706), the 345 Talent Project of Shengjing Hospital, and the clinical research project of Liaoning Diabetes Medical Nutrition Prevention Society (Grant No. LNSTNBYXYYFZXH-RS01B).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-192
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-192). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Letong L, Ling L. Considerations on guidelines for gestational diabetes. Chinese Journal of Practical Internal Medicine 2018;6:38-6.
- Poomalar GK. Changing trends in management of gestational diabetes mellitus. World J Diabetes 2015;6:284-95. [Crossref] [PubMed]
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 2020;369:m1361. [Crossref] [PubMed]
- Akinci B, Celtik A, Genc S, et al. Evaluation of postpartum carbohydrate intolerance and cardiovascular risk factors in women with gestational diabetes. Gynecol Endocrinol 2011;27:361-7. [Crossref] [PubMed]
- Akinci B, Celtik A, Yener S, et al. Prediction of developing metabolic syndrome after gestational diabetes mellitus. Fertil Steril 2010;93:1248-54. [Crossref] [PubMed]
- Akinci B, Celtik A, Yuksel F, et al. Increased osteoprotegerin levels in women with previous gestational diabetes developing metabolic syndrome. Diabetes Res Clin Pract 2011;91:26-31. [Crossref] [PubMed]
- Landon MB, Mele L, Spong CY, et al. The relationship between maternal glycemia and perinatal outcome. Obstet Gynecol 2011;117:218-24. [Crossref] [PubMed]
- Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol 2013;122:406-16. [Crossref] [PubMed]
- Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet 2015;131:S173-211. [Crossref]
- Diabetes in pregnancy: management from preconception to the postnatal period. NICE guideline. Published 25th Feb 2015. Available online: https://www.nice.org.uk/guidance/ng3
- IDF GDM Model of Care: Implementation Protocol: Guidelines for Healthcare Professionals. Available online: https://www.idf.org/e-library/guidelines/77-idf-gdm-model-of-care-implementation-protocol-guidelines-for healthcareprofessionals.html
- Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4227-49. [Crossref] [PubMed]
- Norman RJ, Wang JX, Hague W. Should we continue or stop insulin sensitizing drugs during pregnancy? Curr Opin Obstet Gynecol 2004;16:245-50. [Crossref] [PubMed]
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 2008;358:2003-15. [Crossref] [PubMed]
- Aroda VR, Knowler WC, Crandall JP, et al. Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia 2017;60:1601-11. [Crossref] [PubMed]
- Gutzin SJ, Kozer E, Magee LA, et al. The safety of oral hypoglycemic agents in the first trimester of pregnancy: a meta-analysis. Can J Clin Pharmacol 2003;10:179-83. [PubMed]
- Hellmuth E, Damm P, Mølsted-Pedersen L. Oral hypoglycaemic agents in 118 diabetic pregnancies. Diabet Med 2000;17:507-11. [Crossref] [PubMed]
- Sheehan MT. Polycystic ovarian syndrome: diagnosis and management. Clin Med Res 2004;2:13-27. [Crossref] [PubMed]
- Khattab S, Mohsen IA, Aboul Foutouh I, et al. Can metformin reduce the incidence of gestational diabetes mellitus in pregnant women with polycystic ovary syndrome? Prospective cohort study. Gynecol Endocrinol 2011;27:789-93. [Crossref] [PubMed]
- Nawaz FH, Rizvi J. Continuation of metformin reduces early pregnancy loss in obese Pakistani women with polycystic ovarian syndrome. Gynecol Obstet Invest 2010;69:184-9. [Crossref] [PubMed]
- Eyal S, Easterling TR, Carr D, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos 2010;38:833-40. [Crossref] [PubMed]
- Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition at 2 years of age. Diabetes Care 2011;34:2279-84. [Crossref] [PubMed]
- Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care 2018;6:e000456 [Crossref] [PubMed]
- Carlsen SM, Martinussen MP, Vanky E. Metformin's effect on first-year weight gain: a follow-up study. Pediatrics 2012;130:e1222-6. [Crossref] [PubMed]
- Ijäs H, Vääräsmäki M, Saarela T, et al. A follow-up of a randomised study of metformin and insulin in gestational diabetes mellitus: growth and development of the children at the age of 18 months. BJOG 2015;122:994-1000. [Crossref] [PubMed]
- Xu Q, Xie Q. Long-term effects of prenatal exposure to metformin on the health of children based on follow-up studies of randomized controlled trials: a systematic review and meta-analysis. Arch Gynecol Obstet 2019;299:1295-303. [Crossref] [PubMed]
- Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia 2017;60:1577-85. [Crossref] [PubMed]
- Luciano-Mateo F, Hernández-Aguilera A, Cabre N, et al. Nutrients in Energy and One-Carbon Metabolism: Learning from Metformin Users. Nutrients 2017;9:121. [Crossref] [PubMed]
- Denno KM, Sadler TW. Effects of the biguanide class of oral hypoglycemic agents on mouse embryogenesis. Teratology 1994;49:260-6. [Crossref] [PubMed]
- Lee HY, Wei D, Loeken MR. Lack of metformin effect on mouse embryo AMPK activity: implications for metformin treatment during pregnancy. Diabetes Metab Res Rev 2014;30:23-30. [Crossref] [PubMed]
- Gonzalez CD, Alvariñas J, Bagnes MFG, et al. Metformin and Pregnancy Outcomes: Evidence Gaps and Unanswered Questions. Curr Clin Pharmacol 2019;14:54-60. [Crossref] [PubMed]
- Gilbert C, Valois M, Koren G. Pregnancy outcome after first-trimester exposure to metformin: a meta-analysis. Fertil Steril 2006;86:658-63. [Crossref] [PubMed]
- Bolton S, Cleary B, Walsh J, et al. Continuation of metformin in the first trimester of women with polycystic ovarian syndrome is not associated with increased perinatal morbidity. Eur J Pediatr 2009;168:203-6. [Crossref] [PubMed]
- Bao LX, Shi WT, Han YX. Metformin versus insulin for gestational diabetes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2019; Epub ahead of print. [Crossref] [PubMed]
- Ashoush S, El-Said M, Fathi H, et al. Identification of metformin poor responders, requiring supplemental insulin, during randomization of metformin versus insulin for the control of gestational diabetes mellitus. J Obstet Gynaecol Res 2016;42:640-7. [Crossref] [PubMed]
- Arshad R, Khanam S, Shaikh F, et al. Feto-maternal outcomes and Glycemic control in Metformin versus insulin treated Gestational Diabetics. Pak J Med Sci 2017;33:1182-7. [Crossref] [PubMed]
- Mesdaghinia E, Samimi M, Homaei Z, et al. Comparison of newborn outcomes in women with gestational diabetes mellitus treated with metformin or insulin: a randomised blinded trial. Int J Prev Med 2013;4:327-33. [PubMed]
- Spaulonci CP, Bernardes LS, Trindade TC, et al. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol 2013;209:34.e1-7. [Crossref] [PubMed]
- Ruholamin S, Eshaghian S, Allame Z. Neonatal outcomes in women with gestational diabetes mellitus treated with metformin in compare with insulin: A randomized clinical trial. J Res Med Sci 2014;19:970-5. [PubMed]
- Nachum Z, Zafran N, Salim R, et al. Glyburide Versus Metformin and Their Combination for the Treatment of Gestational Diabetes Mellitus: A Randomized Controlled Study. Diabetes Care 2017;40:332-7. [Crossref] [PubMed]
- Silva JC, Pacheco C, Bizato J, et al. Metformin compared with glyburide for the management of gestational diabetes. Int J Gynaecol Obstet 2010;111:37-40. [Crossref] [PubMed]
- Moore LE, Clokey D, Rappaport VJ, et al. Metformin compared with glyburide in gestational diabetes: a randomized controlled trial. Obstet Gynecol 2010;115:55-9. [Crossref] [PubMed]
- Rai L, Meenakshi D, Kamath A. Metformin--a convenient alternative to insulin for Indian women with diabetes in pregnancy. Indian J Med Sci 2009;63:491-7. [Crossref] [PubMed]
- Kitwitee P, Limwattananon S, Limwattananon C, et al. Metformin for the treatment of gestational diabetes: An updated meta-analysis. Diabetes Res Clin Pract 2015;109:521-32. [Crossref] [PubMed]
- Li G, Zhao S, Cui S, et al. Effect comparison of metformin with insulin treatment for gestational diabetes: a meta-analysis based on RCTs. Arch Gynecol Obstet 2015;292:111-20. [Crossref] [PubMed]
- Amin M, Suksomboon N, Poolsup N, et al. Comparison of glyburide with metformin in treating gestational diabetes mellitus: a systematic review and meta-analysis. Clin Drug Investig 2015;35:343-51. [Crossref] [PubMed]
- Singh KP, Rahimpanah F, Barclay M. Metformin for the management of gestational diabetes mellitus. Aust N Z J Obstet Gynaecol 2015;55:303-8. [Crossref] [PubMed]
- Poolsup N, Suksomboon N, Amin M. Efficacy and safety of oral antidiabetic drugs in comparison to insulin in treating gestational diabetes mellitus: a meta-analysis. PLoS One 2014;9:e109985 [Crossref] [PubMed]
- Feng Y, Yang H. Metformin - a potentially effective drug for gestational diabetes mellitus: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2017;30:1874-81. [Crossref] [PubMed]
- Jiang YF, Chen XY, Ding T, et al. Comparative efficacy and safety of OADs in management of GDM: network meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2015;100:2071-80. [Crossref] [PubMed]
- Gui J, Liu Q, Feng L. Metformin vs insulin in the management of gestational diabetes: a meta-analysis. PLoS One 2013;8:e64585 [Crossref] [PubMed]
- Ijäs H, Vääräsmäki M, Morin-Papunen L, et al. Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG 2011;118:880-5. [Crossref] [PubMed]
- Niromanesh S, Alavi A, Sharbaf FR, et al. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Res Clin Pract 2012;98:422-9. [Crossref] [PubMed]
- Tertti K, Ekblad U, Koskinen P, et al. Metformin vs. insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes Metab 2013;15:246-51. [Crossref] [PubMed]
- Ainuddin J, Karim N, Hasan AA, et al. Metformin versus insulin treatment in gestational diabetes in pregnancy in a developing country: a randomized control trial. Diabetes Res Clin Pract 2015;107:290-9. [Crossref] [PubMed]
- Silva JC, Fachin DR, Coral ML, et al. Perinatal impact of the use of metformin and glyburide for the treatment of gestational diabetes mellitus. J Perinat Med 2012;40:225-8. [Crossref] [PubMed]
- George A, Mathews JE, Sam D, et al. Comparison of neonatal outcomes in women with gestational diabetes with moderate hyperglycaemia on metformin or glibenclamide--a randomised controlled trial. Aust N Z J Obstet Gynaecol 2015;55:47-52. [Crossref] [PubMed]
- Balani J, Hyer S, Johnson A, et al. Pregnancy outcomes after metformin treatment for gestational diabetes: a case-control study. Obstet Med 2012;5:78-82. [Crossref] [PubMed]
- Balani J, Hyer SL, Rodin DA, et al. Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: a case-control study. Diabet Med 2009;26:798-802. [Crossref] [PubMed]
- Goh JE, Sadler L, Rowan J. Metformin for gestational diabetes in routine clinical practice. Diabet Med 2011;28:1082-7. [Crossref] [PubMed]
- Gandhi P, Bustani R, Madhuvrata P, et al. Introduction of metformin for gestational diabetes mellitus in clinical practice: Has it had an impact? Eur J Obstet Gynecol Reprod Biol 2012;160:147-50. [Crossref] [PubMed]
- Tertti K, Ekblad U, Vahlberg T, et al. Comparison of metformin and insulin in the treatment of gestational diabetes: a retrospective, case-control study. Rev Diabet Stud 2008;5:95-101. [Crossref] [PubMed]
- Landi SN, Radke S, Boggess K, et al. Comparative effectiveness of metformin versus insulin for gestational diabetes in New Zealand. Pharmacoepidemiol Drug Saf 2019;28:1609-19. [Crossref] [PubMed]
- Zhu B, Zhang L, Fan YY, et al. Metformin versus insulin in gestational diabetes mellitus: a meta-analysis of randomized clinical trials. Ir J Med Sci 2016;185:371-81. [Crossref] [PubMed]
- Liang HL, Ma SJ, Xiao YN, et al. Comparative efficacy and safety of oral antidiabetic drugs and insulin in treating gestational diabetes mellitus: An updated PRISMA-compliant network meta-analysis. Medicine (Baltimore) 2017;96:e7939 [Crossref] [PubMed]
- Su DF, Wang XY. Metformin vs insulin in the management of gestational diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014;104:353-7. [Crossref] [PubMed]
- Guo L, Ma J, Tang J, et al. Comparative Efficacy and Safety of Metformin, Glyburide, and Insulin in Treating Gestational Diabetes Mellitus: A Meta-Analysis. J Diabetes Res 2019;2019:9804708 [Crossref] [PubMed]
- Butalia S, Gutierrez L, Lodha A, et al. Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta-analysis. Diabet Med 2017;34:27-36. [Crossref] [PubMed]
- Tarry-Adkins JL, Aiken CE, Ozanne SE. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis. PLoS Med 2019;16:e1002848 [Crossref] [PubMed]
- Priya G, Kalra S. Metformin in the management of diabetes during pregnancy and lactation. Drugs Context 2018;7:212523 [Crossref] [PubMed]
- Silva AL, Amaral AR, Oliveira DS, et al. Neonatal outcomes according to different therapies for gestational diabetes mellitus. J Pediatr (Rio J) 2017;93:87-93. [Crossref] [PubMed]
- Zhang SM, Wang YF, Li XQ, et al. Effects of metformin on gestational outcomes and neonates in patients with gestational diabetes mellitus. Laboratory Medicine and Clinic 2017;14:2592-5.
- Chen HB, Gao CY, Lai LS. Effect of metformin on pregnancy outcome in patients with gestational diabetes mellitus. Evaluation and Analysis of Drug-Use in Hospitals of China 2018;18:1632-1633, 1636.
- Geng H, Ding XY, Duan BD. Curative effect of metformin combined with insulin as part in treatment of gestational Diabetes mellitus and the impact on the levels of serum cystatin C and homocysteine. Maternal and Child Health Care of China 2018;33:796-9.
- Wu YY. Effect of metformin hydrochloride combined with insulin on serum cystatin C, homocysteine and maternal and infant outcomes in pregnant women with gestational diabetes mellitus. Diabetes New World 2018;21:76-7.
- Nie JX. Effect of insulin combined with metformin on patients with gestational diabetes and pregnancy curative effect on serum CysC and Hcy levels. Chinese Journal of Practical Medicine 2017;44:35-8.
- Hickman MA, McBride R, Boggess KA, Strauss R. Metformin compared with insulin in the treatment of pregnant women with overt diabetes: a randomized controlled trial. Am J Perinatol 2013;30:483-90. [Crossref] [PubMed]
- Kong LY, Yang HX. Effects of gestational diabetes mellitus on fetal growth rate. Chinese Journal of Perinatal Medicine 2014;(8):521-6.
- Farrar D, Simmonds M, Bryant M, et al. Treatments for gestational diabetes: a systematic review and meta-analysis. BMJ Open 2017;7:e015 [Crossref] [PubMed]
- Bishop KC, Harris BS, Boyd BK, et al. Pharmacologic Treatment of Diabetes in Pregnancy. Obstet Gynecol Surv 2019;74:289-97. [Crossref] [PubMed]
- Greenway F, Wang S, Heiman M. A novel cobiotic containing a prebiotic and an antioxidant augments the glucose control and gastrointestinal tolerability of metformin: a case report. Benef Microbes 2014;5:29-32. [Crossref] [PubMed]
- Sinai Talaulikar V, Tang T, Yasmin E. Role of Metformin in Women's Health: Review of Its Current Place in Clinical Practice and Emerging Indications for Future. Obstet Gynecol Surv 2016;71:307-17. [Crossref] [PubMed]
- Salpeter SR, Greyber E, Pasternak GA, et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2010;CD002967 [Crossref] [PubMed]
- Misbin RI, Green L, Stadel BV, et al. Lactic acidosis in patients with diabetes treated with metformin. N Engl J Med 1998;338:265-6. [Crossref] [PubMed]
- DeFronzo Ralph, Fleming G Alexander, et al. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism Clinical and Experimental 2016;65:20-9. [Crossref] [PubMed]
- Salvatore T, Pafundi PC, Marfella R, et al. Metformin lactic acidosis: Should we still be afraid? Diabetes Res Clin Pract 2019;157:107879 [Crossref] [PubMed]
- Kuan IHS, Savage RL, Duffull SB, et al. The Association between Metformin Therapy and Lactic Acidosis. Drug Saf 2019;42:1449-69. [Crossref] [PubMed]
- Mathieu C. Metformin-associated lactic acidosis: time to let it go? J Diabetes Complications 2015;29:974-5. [Crossref] [PubMed]
- Tehrani FR, Simbar M, Tohidi M, et al. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol 2011;9:39. [Crossref] [PubMed]
- Roos N, Kieler H, Sahlin L, et al. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ 2011;343:d6309. [Crossref] [PubMed]
- Begum MR, Khanam NN, Quadir E, et al. Prevention of gestational diabetes mellitus by continuing metformin therapy throughout pregnancy in women with polycystic ovary syndrome. J Obstet Gynaecol Res 2009;35:282-6. [Crossref] [PubMed]
- Zhao J, Liu X, Zhang W. The Effect of Metformin Therapy for Preventing Gestational Diabetes Mellitus in Women with Polycystic Ovary Syndrome: A Meta-Analysis. Exp Clin Endocrinol Diabetes 2020;128:199-205. [Crossref] [PubMed]
- Zhuo Z, Wang A, Yu H. Effect of metformin intervention during pregnancy on the gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and meta-analysis. J Diabetes Res 2014;2014:381231 [Crossref] [PubMed]
- Tan X, Li S, Chang Y, et al. Effect of metformin treatment during pregnancy on women with PCOS: a systematic review and meta-analysis. Clin Invest Med 2016;39:E120-31. [Crossref] [PubMed]
- Feng L, Lin XF, Wan ZH, et al. Efficacy of metformin on pregnancy complications in women with polycystic ovary syndrome: a meta-analysis. Gynecol Endocrinol 2015;31:833-9. [Crossref] [PubMed]
- Vanky Eszter, Stridsklev Solhild, Heimstad Runa, et al. Metformin Versus Placebo from First Trimester to Delivery in Polycystic Ovary Syndrome: A Randomized, Controlled Multicenter Study. J Clin Endocrinol Metab 2010;95:E448-55. [Crossref] [PubMed]
- National Collaborating Centre for Women's and Children's Health (UK). Diabetes in Pregnancy: Management of Diabetes and Its Complications from Preconception to the Postnatal Period. London: National Institute for Health and Care Excellence (UK); February 2015.
- Mahtab H, Bhowmik B. Diabetes in Pregnancy. J Pak Med Assoc 2016;66:S3-4. [PubMed]
- Seshiah V, Banerjee S, Balaji V, et al. Consensus evidence-based guidelines for management of gestational diabetes mellitus in India. J Assoc Physicians India 2014;62:55-62. [PubMed]
- McElduff A, Cheung NW, McIntyre HD, et al. The Australasian Diabetes in Pregnancy Society consensus guidelines for the management of type 1 and type 2 diabetes in relation to pregnancy. Med J Aust 2005;183:373-7. [Crossref] [PubMed]
- Hughes RC, Gardiner SJ, Begg EJ, et al. Effect of pregnancy on the pharmacokinetics of metformin. Diabet Med 2006;23:323-6. [Crossref] [PubMed]


