
Impact of fentanyl on acute and chronic pain and its side effects when used with epidural analgesia after thoracic surgery in multimodal analgesia: a retrospective cohort study
Introduction
Several postoperative management protocols or guidelines, including for enhanced recovery after surgery, recognize that proper management of postoperative pain is an important factor in patient recovery (1-3). Postoperative analgesia management is essential for early postoperative recovery after thoracic surgery (4). Postoperative pain interferes with patient rehabilitation and affects the length of hospital stay and prognosis (2). In particular, in chest and abdominal surgery, postoperative pain is severe, and inadequate analgesia causes shallow breathing and hinders sputum production, causing postoperative pneumonia (5,6). Although various analgesic methods are implemented, the standard for thoracic surgery is still epidural anesthesia (7,8).
However, in recent years, multimodal analgesia combined with an adjuvant analgesic, non-steroidal anti-inflammatory drugs (NSAIDs), or acetaminophen, is being widely used as postoperative analgesia, and it is no longer necessary to seek a stronger analgesic effect on epidural anesthesia than before (2). Alternatively, the role of epidural anesthesia analgesia in pain management modalities that include proactive administration of NSAIDs or acetaminophen should be discussed. Previous evidence-based clinical research should be updated as the medical environment changes. As many clinical studies have shown, the analgesic effect of epidural anesthesia is enhanced by the addition of opioids to local anesthetics (9,10). However, the addition of opioids to epidural anesthesia increases is associated with side-effects such as nausea and vomiting, pruritus dermatitis, and hypotension (9,10). Considering that these side effects are affected by the patient's background factors, the pros and cons of the analgesic effect and side effects of opioids added to epidural anesthesia should be discussed.
In our hospital, patient-controlled epidural analgesia (PCEA) after thoracic surgery has traditionally been performed with 0.15% ropivacaine along with fentanyl; however, we started using a drug solution without fentanyl to reduce side effects. We hypothesized that changes in this protocol would reduce opioid side effects, but increase in postoperative pain would be modest with multimodal analgesia. In this study, we retrospectively compared the difference in postoperative analgesic effect and the onset of side effects between the two pain management modalities and examined measures to provide high-quality postoperative analgesia. We present the following article in accordance with the STROBE reporting checklist (available at: http://dx.doi.org/10.21037/apm-21-136).
Methods
Study design and ethical statement
This was a retrospective cohort study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Hiroshima University Hospital (No. E-1462-1) and individual consent for this retrospective analysis was waived.
Patients selection
This study is a single-center study conducted at Hiroshima University Hospital. We retrospectively investigated the electronic medical records of 525 patients who underwent thoracic surgery between January 2014 and October 2017. To avoid bias, we chose this period as there was no significant reorganization of members of the thoracic surgery department. Postoperative pain management policies were determined in consultation with the surgeon and anesthesiologist, and there were no changes during this period other than discontinuing the addition of opioids for epidural anesthesia. We selected 358 patients who underwent epidural anesthesia for postoperative analgesia. Among the selected patients, those who underwent thoracic surgery other than lung cancer surgery or video-assisted thoracoscopic surgery (VATS) for lung cancer and those aged below 20 years or above 80 years were excluded from the study. We included 282 patients who met the criteria for the analysis. Next, patients to be analyzed were divided into two groups: a group for which fentanyl was added to epidural anesthesia (group F) and another where fentanyl was not added (group N). Regarding post-thoracotomy pain syndrome (PTPS), we investigated the presence or absence of pain at 2 months after the operation during the outpatient examination. Patients who underwent a second surgery or died by the date of examination were excluded from the study. A flowchart of patient selection process is illustrated in Figure 1.

Post-operative pain management
PCEA was the first choice for postoperative analgesia following thoracic surgery in our hospital, and intravenous patient-controlled analgesia (IVPCA) was chosen when PCEA was not possible owing to the use of oral anticoagulants or a patient’s request. We used a CADD-Legacy® PCA, Model 6300 (Smiths Medical, St Paul, MN, USA) or i-FusorTM Plus (JMS, Hiroshima, Japan) for the PCA infusion pump.
In April 2016, we changed our pain management protocol and started using opioid-free PCEA for postoperative pain management in all cases of thoracic surgery. PCEA chemical solutions and pump settings were as follows: 0.15% ropivacaine with 2 µg/mL fentanyl (until April 2016) or 0.15% ropivacaine alone (after April 2016), background infusion rate of 3 mL/h, demand dose of 2 mL/push, and lockout interval of 15 min. This means that group F corresponds to patients in the period January 2014 to April 2016, and group N corresponds to patients in the period April 2016 to October 2017.
Movement pain visual analog scale (VAS) scores while walking for 0 to 100 mm and rest VAS scores were measured twice daily from day 1 to day 3 after the surgery. As a standard PCEA management protocol, the continuous dose was reduced to 1 mL/h on the 2nd postoperative day, and the catheter was removed in the morning of the 3rd postoperative day. This dosing schedule was modified depending on the patient’s side effects. If the side effects did not improve with symptomatic treatment or follow-up, we reduced the dose of PCEA or discontinued PCEA. We set the observation period up to 3 days after surgery as the observation period for postoperative analgesia.
Regarding the use of adjuvant analgesics, flurbiprofen 50 mg (maximum: up to 3 times a day) or acetaminophen 1,000 mg (maximum: up to 4 times a day; if the bodyweight is less than 50 kg, administered 15 mg per 1 kg of body weight at a time) was intravenously administered on the day of surgery when the patient complained of pain. If the pain continued after the first day after the surgery, regular oral administration of celecoxib 400 mg/day or loxoprofen 180 mg/day was started in addition to the aforementioned dose of intravenous analgesics.
Surveyed factors
We investigated the patients’ background factors, including age, sex, height, body weight, and coexisting diseases, including hypertension, diabetes mellitus, chronic obstructive pulmonary disease, renal dysfunction, smoking history, method of postoperative pain management (PCEA or not), and VAS scores. We also investigated the frequency of postoperative adjuvant analgesic administration, including loxoprofen, celecoxib, and acetaminophen, as well as of side effects, including nausea, vomiting, pruritus, hypotension, and urinary retention. Additionally, we investigated the proportion of patients whose PCEA dose was unexpectedly reduced or whose PCEA was discontinued owing to side effects.
As an evaluation of postoperative rehabilitation, we investigated whether the patient could stand or walk the day after the surgery. That is, we investigated the proportion of patients with delayed ambulation (standing up and walking).
Furthermore, we investigated the presence of PTPS, defined as pain along the thoracotomy scar recurring or persisting for more than 2 months after the surgery.
Statistical analysis
The sample size was calculated using G*Power 3.1.0 (Heinrich Heine University Düsseldorf), and the number of patients required to detect a difference in resting VAS was calculated to be 130 per group with an α error of 0.05 and 1-β error of 0.8. Since the number of thoracic surgery cases was about 100 per year, a 2-year study period was set for each group.
We used univariate analysis to compare the study factors between the two groups with and without fentanyl for PCEA. A Mann-Whitney U test was used for quantitative variables, and a chi-squared test was used for categorical variables for comparison between the two groups.
Pain VAS scores (both resting and movement pain), number of bolus doses, and use of adjuvant analgesics were compared at multiple time points, therefore, we performed Bonferroni correction for adjust for multiplicity. A P value <0.05 was considered to indicate statistical significance. Values are shown as median [interquartile range] or number (%).
Results
A total of 525 patients were included at baseline. Thereafter, 167 patients whose postoperative pain was managed by methods other than PCEA were excluded from the analysis. Additionally, 54 patients who underwent surgery other than VATS and 22 patients below the age of 20 years or above the age of 80 years were excluded from the analysis. Finally, a total of 282 patients were included in the analysis, with 142 in group F and 140 in group N. All patients required adjuvant analgesics during the observation period up to 3 days postoperatively. For PTPS, post-discharge pain follow-up was possible in 277 patients: 138 in group F and 139 in group N. Five patients (one died and four re-surgery) dropped out of PTPS follow-up. A total of 38 patients in group F and 43 in group N were diagnosed with PTPS.
Table 1 shows the background characteristics of the patients. Height and body weight were lower in group F than those in group N; however, there were no differences in other factors.

Full table
Table 2 shows the pain VAS scores during rest and movement, the number of bolus requests for PCEA, and the frequency of use of adjuvant analgesics. Group F had a lower pain VAS score and less bolus requests than group N did. Adjuvant analgesics were administered in all cases with a single dose or regular administration. The frequency of use of adjuvant analgesics in group F was lower than that in group N.
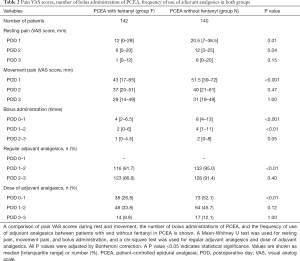
Full table
Table 3 shows the incidence of side effects, unplanned dose reduction and discontinuation of PCEA, and rehabilitation status. Hypotension and pruritus were more frequent in group F than in group N. Unplanned dose reduction and discontinuation of PCEA were more common in group F than in group N (P<0.01). There was no difference in rehabilitation status between the two groups.

Full table
Table 4 shows the patients surveyed for PTPS and PTPS frequency. There was no difference in the frequency of PTPS between the two groups (P=0.53).

Full table
Discussion
We investigated the advantages and disadvantages of adding fentanyl to epidural anesthesia in patients who received PCEA as pain management with multimodal analgesia after thoracic surgery. The addition of fentanyl to PCEA reduced early postoperative pain; however, it increased the frequency of pruritus and hypotension. In contrast, when fentanyl was not added to PCEA, the required amount of adjuvant analgesic increased, but the pain VAS score itself was low, and ambulation was not delayed.
Several studies have reported that the addition of fentanyl to epidural anesthesia reduces postoperative pain (11-13). Moreover, the addition of fentanyl to epidural anesthesia has been reported to reduce the use of adjuvant analgesics (11,12). Similarly, in our study, patients who received fentanyl-PCEA had low postoperative rest and movement pain VAS scores and less use of adjuvant analgesics. It was considered that a stronger analgesic effect could be obtained by adding fentanyl to PCEA. In contrast, the PCEA group that did not receive fentanyl had more resting pain during the study period and movement pain until the second day after surgery; however, their ambulation was not delayed. It was considered that the pain was sufficiently suppressed even in the group which did not receive fentanyl with PCEA owing to the aggressive use of adjuvant analgesics. Additionally, unplanned dose changes and discontinuation of PCEA due to side effects were significantly more frequent in the fentanyl-PCEA group. Considering these results, the pros and cons of adding fentanyl to PCEA should be considered not only for its analgesic effect but also for the side effects of adjuvant analgesics and fentanyl (14-16). Although our study could not investigate the side effects of NSAIDs, these should definitely be considered. The addition of fentanyl to epidural anesthesia is recommended for patients who need to avoid the side effects of NSAIDs, namely gastritis, peptic ulceration, and impaired renal function; however, it is not recommended for patients at high risk for pruritus and hypotension.
Regarding the details of the change in side effects when fentanyl was added to PCEA, hypotension, and pruritus were significantly more common in the fentanyl PCEA group. Hypotension owing to epidural anesthesia is caused by blockage of the sympathetic nerve by a local anesthetic (17,18). In our study, local anesthetic concentrations were the same in both groups; therefore, we expected that hypotension would be more frequent in the PCEA group that did not receive fentanyl as they had a high bolus frequency. However, the frequency of hypotension was higher in the PCEA group that received fentanyl. It has been reported that the higher the concentration of fentanyl added to epidural anesthesia, the higher the frequency of side effects such as hypotension, nausea, and pruritus, and in terms of PCEA, fentanyl may have a greater effect on hypotension than local anesthesia do (19).
As for postoperative nausea and vomiting (PONV), the incidence of postoperative epidural analgesia is reported to be 14–24.8%, and in our study, the frequency was similar (20). Incidence of PONV was 17% in the fentanyl PCEA group and 12% in the fentanyl-free PCEA group; the difference was not significant. PONV occurs more in frequently in women and nonsmokers (21,22). In our study, the absence of increase in the incidence of PONV could be attributed to the following reasons: the advanced age of the patients studied, the large proportion of male patients, and the majority of smokers (23).
Opioids are known to cause pruritus (24). Furthermore, the frequency of pruritus varies depending on the administration route of opioids, and pruritus occurs in 30–100% of cases of epidural administration (25). In our study, pruritus was observed in approximately 40% of cases in which fentanyl was added to PCEA. Additionally, pruritus resulted in dose reduction and discontinuation of PCEA in 4.2% of cases, which was relatively common. In situations where adjuvant analgesics provide adequate analgesia, pruritus may have a negative impact on the continuation of PCEA.
For PTPS, we assessed the patient’s post-discharge pain. PTPS occurs in approximately 50% of patients after open chest surgery, which is consistent with our findings (26,27). Nerve blocks such as epidural anesthesia have been reported to be useful in the prevention of PTPS; however, no efficacy has been reported for opioids (28). Similarly, in our study, the incidence of PTPS did not differ with or without the addition of fentanyl to PCEA. We believe that it is not necessary to add fentanyl to epidural anesthesia to prevent PTPS.
Our research has some limitations due to retrospective study design. First, this research was conducted in a single university hospital and did not perform multivariable analysis. Therefore, our results may not be generalized to other hospitals. Second, the biases that can occur in cohort studies include confounding bias, selection bias, and information bias, and some of these biases may also occur in our study. We conducted a survey of patients who underwent epidural anesthesia and chest surgery. We believe that the sampling bias is minimized as the epidural anesthesia protocol changes have been applied to all cases since April 2016. Furthermore, to avoid bias, we chose a period with no significant reorganization of thoracic surgery members and no change in postoperative management in the ward. In addition, research subjects include lead-time bias. We could not examine the details of the surgical procedure in our research. In our study data, the pain was stronger in the group without fentanyl added to PCEA after 2016. Assuming that the surgical technique is becoming less invasive over time, the pain should be less in the group later in the study period. Our study may underestimate the difference in pain between the two groups.
Conclusions
In PCEA after thoracic surgery, when fentanyl was added to a local anesthetic, the pain VAS score decreased; however, the incidence of hypotension and pruritus increased. Currently, the use of multimodal analgesia is widespread, and sufficient analgesia can be obtained without adding fentanyl to epidural anesthesia. Therefore, adding fentanyl to PCEA should be decided after considering the side effects and the restrictions on the use of adjuvant analgesics for each patient.
Acknowledgments
The authors acknowledge the support of the postoperative pain management team of the Hiroshima University Hospital.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at: http://dx.doi.org/10.21037/apm-21-136
Data Sharing Statement: Available at: http://dx.doi.org/10.21037/apm-21-136
Peer Review File: Available at: http://dx.doi.org/10.21037/apm-21-136
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/apm-21-136). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Hiroshima University Hospital (No. E-1462-1) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chemali ME, Eslick GD. A meta-analysis: postoperative pain management in colorectal surgical patients and the effects on length of stay in an enhanced recovery after surgery (ERAS) setting. Clin J Pain 2017;33:87-92. [Crossref] [PubMed]
- American Society of Anesthesiologists Task Force on Acute Pain Management. Practice Guidelines for Acute Pain Management in the Perioperative Setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116:248-73. [Crossref] [PubMed]
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative council. J Pain 2016;17:131-57. [Crossref] [PubMed]
- Mehran RJ, Martin LW, Baker CM, et al. Pain management in an enhanced recovery pathway after thoracic surgical procedures. Ann Thorac Surg 2016;102:e595-6. [Crossref] [PubMed]
- Bernard A, Ferrand L, Hagry O, et al. Identification of prognostic factors determining risk groups for lung resection. Ann Thorac Surg 2000;70:1161-7. [Crossref] [PubMed]
- Schussler O, Alifano M, Dermine H, et al. Postoperative pneumonia after major lung resection. Am J Respir Crit Care Med 2006;173:1161-9. [Crossref] [PubMed]
- Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107:1026-40. [Crossref] [PubMed]
- Messina M, Boroli F, Landoni G, et al. A comparison of epidural vs. paravertebral blockade in thoracic surgery. Minerva Anestesiol 2009;75:616-21. [PubMed]
- Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006;96:418-26. [Crossref] [PubMed]
- Okajima H, Tanaka O, Ushio M, et al. Ultrasound-guided continuous thoracic paravertebral block provides comparable analgesia and fewer episodes of hypotension than continuous epidural block after lung surgery. J Anesth 2015;29:373-8. [Crossref] [PubMed]
- Berti M, Casati A, Fanelli G, et al. 0.2% ropivacaine with or without fentanyl for patient-controlled epidural analgesia after major abdominal surgery: a double-blind study. J Clin Anesth 2000;12:292-7. [Crossref] [PubMed]
- Khanna A, Saxena R, Dutta A, et al. Comparison of ropivacaine with and without fentanyl vs bupivacaine with fentanyl for postoperative epidural analgesia in bilateral total knee replacement surgery. J Clin Anesth 2017;37:7-13. [Crossref] [PubMed]
- Matsota P, Batistaki C, Apostolaki S, et al. Patient-controlled epidural analgesia after caesarean section: levobupivacaine 0.15% versus ropivacaine 0.15% alone or combined with fentanyl 2 µg/ml: a comparative study. Arch Med Sci 2011;7:685-93. [Crossref] [PubMed]
- Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: rationale for use in severe postoperative pain. Br J Anaesth 1991;66:703-12. [Crossref] [PubMed]
- Mather LE. Do the pharmacodynamics of the nonsteroidal anti-inflammatory drugs suggest a role in the management of postoperative pain? Drugs 1992;44:1-12; discussion 13. [Crossref] [PubMed]
- Dolin SJ, Cashman JN. Tolerability of acute postoperative pain management: nausea, vomiting, sedation, pruritus, and urinary retention. Evidence from published data. Br J Anaesth 2005;95:584-91. [Crossref] [PubMed]
- Scott DA, Blake D, Buckland M, et al. A comparison of epidural ropivacaine infusion alone and in combination with 1, 2, and 4 microg/mL fentanyl for seventy-two hours of postoperative analgesia after major abdominal surgery. Anesth Analg 1999;88:857-64. [Crossref] [PubMed]
- Hogan QH, Stekiel TA, Stadnicka A, et al. Region of epidural blockade determines sympathetic and mesenteric capacitance effects in rabbits. Anesthesiology 1995;83:604-10. [Crossref] [PubMed]
- Otton PE, Wilson EJ. The cardiocirculatory effects of upper thoracic epidural analgesia. Can Anaesth Soc J 1966;13:541-9. [Crossref] [PubMed]
- Guay J, Nishimori M, Kopp SL. Epidural local anesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting, and pain after abdominal surgery: a Cochrane review. Anesth Analg 2016;123:1591-602. [Crossref] [PubMed]
- Apfel CC, Philip BK, Cakmakkaya OS, et al. Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? Anesthesiology 2012;117:475-86. [Crossref] [PubMed]
- Apfel CC, Läärä E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999;91:693-700. [Crossref] [PubMed]
- Brattwall M, Warrén Stomberg M, Rawal N, et al. Postoperative impact of regular tobacco use, smoking or snuffing, a prospective multi-center study. Acta Anaesthesiol Scand 2010;54:321-7. [Crossref] [PubMed]
- Gan TJ, Ginsberg B, Glass PS, et al. Opioid-sparing effects of a low-dose infusion of naloxone in patient-administered morphine sulfate. Anesthesiology 1997;87:1075-81. [Crossref] [PubMed]
- Szarvas S, Harmon D, Murphy D. Neuraxial opioid-induced pruritus: a review. J Clin Anesth 2003;15:234-9. [Crossref] [PubMed]
- Bertrand PC, Regnard JF, Spaggiari L, et al. Immediate and long-term results after surgical treatment of primary spontaneous pneumothorax by VATS. Ann Thorac Surg 1996;61:1641-5. [Crossref] [PubMed]
- Katz J, Jackson M, Kavanagh BP, et al. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain 1996;12:50-5. [Crossref] [PubMed]
- Park SK, Yoon S, Kim BR, et al. Pre-emptive epidural analgesia for acute and chronic post-thoracotomy pain in adults: a systematic review and meta-analysis. Reg Anesth Pain Med 2020;45:1006-16. [Crossref] [PubMed]


