The effect of sedentary time on the results of exercise therapy in patients with peripheral arterial disease complicated with type 2 diabetes
Introduction
Peripheral artery disease (PAD) is a progressive condition that is characterized by reduced blood flow to the heart from the lower limbs (1). PAD is characterized by limb ischemia-induced claudication or movement that leads to severe pain, walking disorders, and reduced quality of life (2). PAD is associated with an increased risk of cardiovascular disease and death. The risk of death from all causes in the elderly with claudication is 2–3.5 times higher than that of ordinary people (3). The main treatment for symptomatic PAD patients is supervised exercise therapy (SET) aimed at improving physical function, mobility, and quality of life.
One of the main risk factors for PAD is diabetes. Epidemiological studies have shown that 20–30% of adult diabetic patients have PAD (4-7); PAD combined with diabetes puts individuals at a greater risk compared to those with a single disease (8). SET has been shown to be an effective therapy to improve pain relief rate and walking distance (9). It has been reported that some patients, especially those with PAD complicated by diabetes, may be slow to respond to exercise therapy (10). It is not clear why different patients respond inconsistently to exercise, however some have suggested that during exercise, diabetes can promote the reduction of blood volume expansion and impaired skeletal muscle oxygenation (11). Furthermore, dehydration in the elderly may also affect PAD symptoms. Together, these factors affect the benefits of exercise in PAD patients with diabetes.
One behavioral factor that can cause unresponsiveness is a sedentary lifestyle. Studies have shown that compared with regular exercise and physical activity, sedentary behavior is associated with an increased risk of cardiovascular disease (12). People who are often sedentary have almost no changes in their daily activity patterns, and patients with PAD and type 2 diabetes are particularly accustomed to being sedentary (13). Importantly, the longer the sedentary time, the faster the peak walking distance decreases (14).
Given the harmful effects of sedentariness on health, this study aims to evaluate the efficacy of SET in PAD patients, and to evaluate the effect of changes in sedentary time on the results of SET.
We present the study in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-773).
Methods
Research subject
One hundred PAD patients who were treated in our hospital from January 2019 to October 2020 were included in this study. Inclusion criteria: (I) patients aged over 60 years old; (II) patients diagnosed with PAD; and (III) patients that received SET therapy for PAD. Exclusion criteria: (I) patients with type 1 diabetes; and (II) patients with incurable wounds who could not participate in SET therapy. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Sichuan Provincial People’s Hospital (No.: 2020101) and informed consent was taken from all the patients.
Data collection
The basic clinical data of patients, including age, gender, body mass index (BMI), hypertension, smoking, and ankle brachial index (ABI) were collected. The patients completed SET treatment 2–3 times a week for 12 weeks, which mainly involved repeated walking exercises on a treadmill. The course of treatment was formulated according to the treatment protocol for PAD patients, and individualized adjustments were made according to the specific needs of each patient. The intensity of exercise gradually increased over the 12-week period. Although treadmill walking was the primary exercise, patients were also guided to train their arm and leg strength according to their actual situation, and they also received full-body lying down training.
The patients were asked to wear a measuring device that objectively recorded sedentary time and physical activity, and were required to complete physical function and fall risk assessments at three-time points: baseline, 6 weeks, and 12 weeks. Sedentary behavior was defined as the energy expenditure of sitting, leaning, or lying down in any waking state ≤1.5% (15).
The body function of patients was assessed using the objective 6-minute walk test (6MWT) and the Short Physical Performance Battery (SPPB) (16,17). At the same time, two self-rating scales were used for evaluation, including the Walking Impairment Questionnaire (WIQ) and Patient-Reported Outcomes Measurement Information System (PROMIS) (17,18). The WIQ included three sections: walking distance, walking speed, and climbing the stairs. The score ranged from 0 to 100 points, with 100 points representing no impairment of body function, and 0 points representing complete impairment. PROMIS included 15 items to evaluate the difficulty of the patient’s mobility, such as standing unsupported for 30 minutes, jumping up and down, and tiptoeing. The score ranged from 0 to 100, with higher scores denoting better body function.
Statistical analysis
Descriptive statistics were used to summarize the baseline characteristics of the population as well as the change in sedentary time at 6 and 12 weeks (compared with the baseline). The t-test was used to assess the changes in the PAD-only group and the PAD complicated with the type 2 diabetes (T2DM) group. After adjusting for other key confounding variables, such as age, gender, and smoking status, linear regression analysis was used to evaluate the effects of changes in sedentary time on the total distance of the 6MWT. SPSS (version 25, IBM Corp., Armonk, New York) was used for statistical analysis, and P<0.05 was considered to indicate a statistically significant difference.
Results
Basic clinical data of the research subjects
Among the 100 subjects enrolled in this study, there were 47 patients with PAD complicated with T2DM. Of these patients, 46.8% were males, with an average age of 62.1±8.8 years, a BMI of 29.5±5.1 kg/m2, and an ABI of 0.66±0.21. Smoking patients accounted for 44.7%, while patients with hypertension, hyperlipidemia, coronary heart disease, stroke, carotid endarterectomy, lower extremity revascularization accounted for 95.7%, 97.9%, 63.6%, 16.3%, 17.0%, and 57.4%, respectively. There was no significant statistical difference between the baseline clinical data of PAD patients complicated with T2DM and PAD-only patients (P>0.05) (Table 1).
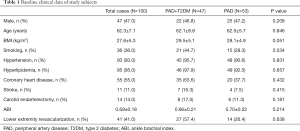
Full table
Patient treatment completion status and outcome
After 12 weeks of SET in 100 patients with PAD, physical function was evaluated and compared with the baseline data. It was found that after 12 weeks of treatment, the total SPPB score of the patients increased from a baseline of 9.3±2.7 to 10.1±2.3 (P=0.025). In the 6MWT, the normal walking distance of patients increased from 108.9±26.8 to 148.9±29.5 m (P<0.001), and the total walking distance increased from 322.5±93.4 m to 348.5±86.1 m (P=0.042). Also, the metabolic equivalent on the treadmill was increased from 2.6±0.7 to 3.9±1.4 (P<0.001). WIQ survey results showed that the walking distance of PAD patients increased from 35.7±24.7 to 43.6±28.7 (P=0.038) and the speed increased from 33.7±22.1 to 44.2±21.6 m/s (P=0.001) (Table 2).

Full table
Changes in overall sedentary time and physical activity
We also compared the 6- and 12-week sedentary time and physical activity level of PAD patients with the baseline, and found that the proportion of time that patients spent with mild physical activity at 6 weeks increased by 20%±10% (P=0.003), and the average length of sedentary time per day was reduced by 6.5±2.8 minutes (P=0.008), or by 3.1%±2.1% (P=0.04). The number of rests every day during sedentary periods increased by 0.2±0.1 times (P=0.003) and the average rest time increased by 4.8±3.1 min (P=0.009). Compared with the baseline, the proportion of time that patients spent engaged in light and moderate physical activity at 12 weeks increased by 10%±3% (P=0.007) and 20%±10% (P=0.006), respectively, and the average sedentary time per day was reduced by 6.8±3.1 minutes (P=0.03), or by 3.6%±1.8% (P=0.005). Furthermore, the number of rests per day during sedentary periods was increased by 0.6±0.2 times (P=0.007) (Table 3).

Full table
Comparison of changes in body function between PAD-only patients and PAD patients complicated with T2DM
A subgroup analysis based on whether the patients were complicated with T2DM found that in the 6MWT, there was no significant statistical difference in the improvement of total walking distance between the two groups of patients. Meanwhile, patients in the PAD complicated with T2DM group had a markedly better increase in normal walking distance than patients in the PAD-only group (P=0.04) (Figure 1).

Discussion
In this study, we found that the change of sedentary time has a significant impact on the effect of PAD patients treated with SET for 12 weeks. Compared with PAD-only patients, those with PAD complicated with T2DM had a more significant improvement in their physical function after SET.
In 2007, the American Sports Medicine Association and American Heart Association jointly recommended 3 months of SET (30 minutes of moderate-intensity activity 5 days a week or 20 minutes of vigorous activity 3 days a week) (19). Consistent with this, the physical function of patients in our study was significantly improved after undergoing SET. Furthermore, we also found that the improvement in normal walking distance of patients with T2DM was greater than that of patients without T2DM, which is consistent with the findings of Ubels et al. (20).
At the same time, we found that the change in sedentary time was consistent with the patient’s SET effect. Compared with the baseline data, the patients’ sedentary time at 6 and 12 weeks decreased significantly, while the physical activity function increased during that time. Sedentariness is a common and poor behavior in the modern economy. Studies have shown that people who sit for prolonged periods of time have a significant increase in cardiovascular risk (up to 30%) (21). This is because in the process of sitting for an extended period, the metabolic equivalent of the body decreases, the activity level is reduced, and the efficiency of blood circulation is significantly reduced. These changes can easily cause blood stasis, thereby increasing the risk of atherosclerosis as well as the long-term risk of cardiovascular death (22). SET is a treatment strategy aimed at increasing physical activity, which can help PAD patients to recover their physical function to varying degrees (23). Actively altering the sedentary lifestyle during SET can effectively increase the degree of physical activity and simultaneously reduce the risks and harms caused by sedentariness, thereby ultimately enhancing physical function more effectively.
Type 2 diabetes is a common endocrine and metabolic disease. At present, the number of T2DM patients in China has reached 130 million, and this number continues to increase (23). Type 2 diabetes is an important risk factor for cardiovascular disease. Studies have shown that the risk of cardiovascular events in patients with type 2 diabetes is 63% higher than that of healthy people (24). Sitting for prolonged periods of time is a common lifestyle in patients with type 2 diabetes, which has an important impact on the progression and complications of T2DM. In this study, the subgroup analysis showed that the effect of SET in patients with PAD complicated with T2DM was superior to that of PAD-only patients. This may be due to the fact that SET can markedly increase the degree of physical activity in T2DM patients, and the change of sedentary time has a greater influence on the metabolism and lifestyle of T2DM patients, thus resulting in more obvious benefits.
In this study, the SPPB objective evaluation index was used to evaluate SET in patients, and the WIQ was also used for autonomous evaluation. The results of the two evaluation methods showed consistency, lending sufficient support for the reliability of the findings in this study. However, this study also had certain limitations that should be noted. Firstly, the sample size of this study was small, and thus the conclusions need to be further verified in a larger sample population. Secondly, although we conducted a subgroup analysis based on whether T2DM was involved, we did not adjust for the duration of T2DM in the patients, and more studies are therefore needed for further validation of our findings.
In summary, the reduction in sedentary time can significantly improve the effect of SET in patients with PAD complicated with T2DM. Compared with PAD-only patients, the improvement in patients with T2DM is more significant.
Acknowledgments
Funding: This work was supported by the Sichuan Provincial Department of Science and Technology Project (2020YFS0405).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-773
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-773
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-773). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Sichuan Provincial People’s Hospital (No.: 2020101) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oka RK, Sanders MG. The impact of type 2 diabetes and peripheral arterial disease on quality of life. J Vasc Nurs 2005;23:61-6. [Crossref] [PubMed]
- Mueller T, Hinterreiter F, Luft C, et al. Mortality rates and mortality predictors in patients with symptomatic peripheral artery disease stratified according to age and diabetes. J Vasc Surg 2014;59:1291-9. [Crossref] [PubMed]
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2017;135:e726-79. [PubMed]
- Treat-Jacobson D, McDermott MM, Bronas UG, et al. Optimal exercise programs for patients with peripheral artery disease. Circulation 2019;139:e10-33. [Crossref] [PubMed]
- Beks PJ, Mackaay AJ, de Neeling JN, et al. Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: the Hoorn study. Diabetologia 1995;38:86-96. [Crossref] [PubMed]
- Elhadd TA, Robb R, Jung RT, et al. Pilot study of prevalence of asymptomatic peripheral arterial occlusive disease in patients with diabetes attending a hospital clinic. Pract Diabetes Int 1999;16:163-6. [Crossref]
- Hirsch AT, Criqui MH, Treat-Jacobson DJ, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286:1317-24. [Crossref] [PubMed]
- Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol 2006;47:921-9. [Crossref] [PubMed]
- Vinik AI, Vinik EJ, Colberg SR, et al. Falls risk in older adults with type 2 diabetes. Clin Geriatr Med 2015;31:89-99. [Crossref] [PubMed]
- Lane R, Ellis B, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD000990 [PubMed]
- Parmenter BJ, Raymond J, Dinnen P, et al. A systematic review of randomized controlled trials: walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis 2011;218:1-12. [Crossref] [PubMed]
- Gardner AW, Parker DE, Montgomery PS, et al. Diabetic women are poor responders to exercise rehabilitation in the treatment of claudication. J Vasc Surg 2014;59:1036-43. [Crossref] [PubMed]
- Mohler ER, Lech G, Supple GE, et al. Impaired exerciseinduced blood volume in type 2 diabetes with or without peripheral arterial disease measured by continuous-wave near-infrared spectroscopy. Diabetes Care 2006;29:1856-9. [Crossref] [PubMed]
- Mason McClatchey P, Bauer TA, Regensteiner JG, et al. Dissociation of local and global skeletal muscle oxygen transport metrics in type 2 diabetes. J Diabetes Complications 2017;31:1311-7. [Crossref] [PubMed]
- Sesti G, Antonelli Incalzi R, Bonora E, et al. Management of diabetes in older adults. Nutr Metab Cardiovasc Dis 2018;28:206-18. [Crossref] [PubMed]
- Fernández S, Parodi JC, Moscovich F, et al. Reversal of lower-extremity intermittent claudication and rest pain by hydration. Ann Vasc Surg 2018;49:1-7. [Crossref] [PubMed]
- Hageman D, Gommans LN, Scheltinga MR, et al. Effect of diabetes mellitus on walking distance parameters after supervised exercise therapy for intermittent claudication: a systematic review. Vasc Med 2017;22:21-7. [Crossref] [PubMed]
- Lyu X, Li S, Peng S, et al. Intensive walking exercise for lower extremity peripheral arterial disease: a systematic review and meta-analysis. J Diabetes 2016;8:363-77. [Crossref] [PubMed]
- Edmonds M. Vascular disease in the lower limb in type 1 diabetes. Cardiovasc Endocrinol Metab 2019;8:39-46. [Crossref] [PubMed]
- Zander E, Heinke P, Reindel J, et al. Peripheral arterial disease in diabetes mellitus type 1 and type 2: are there different risk factors? Vasa 2002;31:249-54. [Crossref] [PubMed]
- Allen JD, Stabler T, Kenjale AA, et al. Diabetes status differentiates endothelial function and plasma nitrite response to exercise stress in peripheral arterial disease following supervised training. J Diabetes Complications 2014;28:219-25. [Crossref] [PubMed]
- van Pul KM, Kruidenier LM, Nicolai SP, et al. Effect of supervised exercise therapy for intermittent claudication in patients with diabetes mellitus. Ann Vasc Surg 2012;26:957-63. [Crossref] [PubMed]
- Stamatakis E, Hamer M, Dunstan DW. Screen-based entertainment time, all-cause mortality, and cardiovascular events: population-based study with ongoing mortality and hospital events follow-up. J Am Coll Cardiol 2011;57:292-9. [Crossref] [PubMed]
- Thorp AA, Healy GN, Owen N, et al. Deleterious associations of sitting time and television viewing time with cardiometabolic risk biomarkers: Australian Diabetes, Obesity and Lifestyle (AusDiab) study 2004-2005. Diabetes Care 2010;33:327-34. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



