
The effect of combined supplementation with vitamin D and omega-3 fatty acids on blood glucose and blood lipid levels in patients with gestational diabetes
Introduction
Gestational diabetes (GDM) refers to diabetes diagnosed for the first time during pregnancy, and accompanied by any carbohydrate intolerance and impaired insulin metabolism (1). According to diagnostic criteria and pregnancy age, GDM affects 1–14% of pregnancies (2). GDM has an important impact on the health of pregnant women and their offspring, and can easily induce a series of chronic metabolic diseases, dystocia and neonatal diseases (3-5). Also, hyperinsulinemia and hyperglycemia during pregnancy may not be conducive to the growth and development of the offspring, which has an inhibitory effect on the growth of the fetal period and easily induce neonatal inflammation and diabetes (6-8).
Previous studies have shown that compared with healthy pregnant women, GDM patients have lower vitamin D and omega-3 fatty acids (9,10). However, there are currently no studies that fully analyze the effects of vitamin D supplements and omega-3 fatty acids on metabolism (11,12). Also, the effect of supplementing vitamin D or omega-3 fatty acids alone in women with GDM is uncertain. Studies have shown that vitamin D at a dose of 50,000 IU every 3 weeks for 6 weeks can improve blood glucose, total cholesterol, and low-density lipoprotein (LDL) cholesterol levels in GDM women, however it does not improve insulin resistance (13). Also, after 6 weeks of daily intake of 1,000 mg of omega-3 fatty acids, it was observed that insulin resistance in GDM subjects was significantly improved, but there was no change in blood glucose, insulin sensitivity, and blood lipid levels (14). Another study by Baidal et al. showed that high-dose omega-3 fatty acids and high-dose vitamin D3 treatment improved homeostasis model assessment of beta cell (HOMA-β) in patients with type 1 new-onset diabetes (15).
Therefore, this study aimed to investigate the effects of vitamin D and omega-3 fatty acids on glucose and blood lipid metabolism in GDM women.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-1018).
Methods
Research object
One hundred and fifty patients with GDM between 18 and 40 years old who were admitted to our hospital from May 2019 to December 2020 were included. According to the guidelines of the American Diabetes Association (16), GDM is defined as no previous history of diabetes, and any of the following criteria is met during pregnancy: (I) fasting blood glucose (FBG) ≥5.1 mmol/L; (II) oral glucose tolerance test (OGTT)-1 h blood glucose ≥10.0 mmol/L; (III) OGTT-2 h blood glucose ≥8.5 mmol/L. The exclusion criteria were as follows: (I) patients who were using insulin; (II) those with placental abruption; (III) patients with pre-eclampsia; (IV) patients with eclampsia; (V) those with hypothyroidism or hyperthyroidism; (VI) smokers; and (VII) patients with kidney or liver disease. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Haikou Hospital of the Maternal and Child Health (No. 2018281) and informed consent was taken from all the patients.
Data collection
The patients were divided into test and control groups according to whether they took vitamin D and omega-3 fatty acids. The test group took 40,000 IU of vitamin D and 8,000 mg of omega-3 fatty acids twice a day. Comparative analysis of the changes in blood glucose and blood lipid levels of the two groups of patients was performed after 6 weeks. The clinical and laboratory data of the research subjects were collected, including gender, age, body mass index (BMI), FBG, fasting insulin, triglyceride (TG), high-density lipoprotein (HDL), LDL, very-low-density lipoprotein (VLDL), and total cholesterol. Also, the homeostasis model assessment of insulin resistance (HOMA-IR) and HOMA-β were calculated.
Statistical methods
Continuous variables were expressed as mean ± standard deviation, and categorical variables are expressed as the number of cases (percentage). The differences between groups were compared using the t-test, and the chi-square test was used to assess percentage differences. Repeated measures variance was used to analyze the effects of vitamin D and omega-3 fatty acids on insulin metabolism markers and blood lipid profiles. The mother’s age and baseline BMI were adjusted to avoid potential confounding effects. SAS software (version 9.4, North Carolina State University, USA) was used to analyze the data, and the difference was considered statistically significant when P<0.05.
Results
Clinical data of study subjects
Among the 150 patients with GDM included in the study, 80 patients took vitamin D and omega-3 fatty acids. The average age of these 80 patients was 31.5±4.1 years, the height was 163.7±3.8 cm, and the baseline weight was 73.8±3.8 kg. After taking vitamin D and omega-3 fatty acids for 6 weeks, the body weight was 75.8±3.6 kg, the body weight change was 2.0±0.9 kg, and the baseline BMI, BMI, and BMI change after 6 weeks were 28.9±3.2 kg/m2, 29.3±3.5 kg/m2, and 0.4±0.1 kg/m2, respectively (Table 1). There were no statistical differences (P>0.05) in the clinical data between the test and control groups.
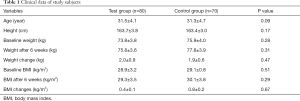
Full table
Baseline glucose and lipid metabolism of the study subjects
The subjects’ baseline glucose and lipid metabolism data showed that the average FBG, fasting insulin, HOMA-IR, and HOMA-β of the experimental group were 5.6±0.9 mmol/L, 13.5±4.6 uIU/mL, 3.2±1.1, and 47.8±16.3, respectively. The average levels of TGs, total cholesterol, LDL, HDL, and VLDL were 2.7±1.2 mmol/L, 6.3±2.1 mmol/L, 4.2±1.8 mmol/L, 1.3±0.9 mmol/L, and 0.92±0.11 mmol/L, respectively (Table 2). No significant statistical differences were observed between the baseline levels of glucose and lipid metabolism between the test and control groups (all P>0.05).
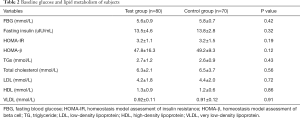
Full table
Changes in glucose and lipid metabolism after using vitamin D and omega-3 fatty acids for 6 weeks
After using vitamin D and omega-3 fatty acids for 6 weeks, the FBG (–0.5±0.2 vs. 0.8±0.1 mmol/L), fasting insulin (–1.9±1.1 vs. 2.3±1.2 uIU/ mL), HOMA-IR (–0.4±0.2 vs. 0.9±0.5), TGs (–0.5±0.2 vs. 0.4±0.1), total cholesterol (–0.8±0.6 vs. 2.8±0.9), LDL (–1.4±0.6 vs. 1.8±0.4), and VLDL (–0.05±0.02 vs. 0.08±0.03) of the test group were markedly decreased compared to the control group (all P<0.05). As shown in Table 3, HOMA-β (0.5±0.2 vs. –0.1±0.3) was significantly improved (P<0.05). However, there were no notable differences in the changes of HDL between the test and control groups (P>0.05).

Full table
The effect of using vitamin D and omega-3 fatty acids for 6 weeks on glucose and lipid metabolism
After adjusting for baseline age and weight, we found that the test group’s FBG, fasting insulin, HOMA-IR, TGs, total cholesterol, LDL, and VLDL were decreased by 0.3±0.2 mmol/L, 1.0±0.6 uIU/mL, 0.2±0.1, 0.3±0.1 mmol/L, 0.5±0.2 mmol/L, 1.1±0.4 mmol/L, and 0.03±0.01 mmol/L, respectively, however HOMA-β was improved by 0.4±0.1. Compared with the placebo group, the test group’s FBG, insulin, HOMA-IR, TGs, total cholesterol, LDL, and VLDL all decreased significantly and HOMA-β was markedly improved, however there was no statistically significant difference in the change of HDL (P>0.05) (Table 4).

Full table
Discussion
Vitamin D is an essential vitamin for the human body. It is involved in regulating calcium and phosphorus metabolism, as well as maintaining healthy bone mineralization (17). Experimental studies have shown that 1,25-dihydroxy vitamin D [1,25(OH)2D], the active form of vitamin D, plays an important role in the stability of the innate and adaptive immune systems, as well as the stability of endothelial cell membranes. The relationship between low serum 25-hydroxyvitamin D levels and increased risk of various immune-related diseases and disorders has been observed, including psoriasis, type 1 diabetes, multiple sclerosis, rheumatoid arthritis, tuberculosis, sepsis, and respiratory tract infections, etc. (18-22). Omega-3 fatty acids are an important component of cell membranes and have antioxidant effects (23,24). Previous studies have found that patients with GDM are often prone to vitamin D and omega-3 fatty acid deficiencies, which may be related to the increased demand during pregnancy and the development of diabetes (25). During pregnancy, hyperglycemia and hyperinsulinemia may lead to permanent diabetes and neonatal complications, including macrosomia and hyperbilirubinemia (26).
This study reported, for the first time, the effect of combined vitamin D and omega-3 fatty acid supplementation on blood sugar. After 6 weeks of supplementation with vitamin D and omega-3 fatty acids in GDM patients, FBG, insulin, HOMA-IR, TGs, total cholesterol, LDL, and VLDL were all significantly decreased, while HOMA-β was dramatically improved. Studies have shown that improving insulin resistance may help to reduce maternal mortality and neonatal complications (27). Consistent with previous studies, this study found that supplementation with vitamin D and omega-3 fatty acids can considerably reduce blood sugar levels (28). Vitamin D is closely related to calcium and phosphorus metabolism. It can affect blood sugar by up-regulating insulin receptor gene expression and transcription (29).
Vitamin D and omega-3 fatty acids may be more effective in treating patients with GDM than a single supplement. In affecting islet blood sugar, omega-3 fatty acids and vitamin D may have a synergistic effect (30). Also, supplementing with omega-3 fatty acids may lead to increased vitamin D content. An et al. observed that, compared with the control group, the vitamin D 1,25(OH)2D levels of dialysis patients who took omega-3 fatty acids without vitamin D increased significantly after 3 months compared with the baseline examination (31). Studies have found that vitamin D deficiency and inflammation alleviation could reduce insulin resistance (32).
This study shows that supplementation with vitamin D and omega-3 fatty acids in GDM patients for 6 weeks can significantly reduce serum TGs, LDL, and VLDL cholesterol, which is consistent with the findings of Davis et al., which indicated the total cholesterol and LDL cholesterol were decreased in healthy people who supplemented with vitamin D and omega-3 fatty acids for 18 months (33). Furthermore, a previous study showed that the increase in maternal lipids was related to complications such as macrosomia, with 36 cases of preeclampsia and 37 cases of preterm delivery (34). However, this study lacked consideration of baseline levels and the specific effects of the subjects themselves. Vitamin D intake may improve lipid distribution and improve insulin sensitivity by increasing calcium absorption (35). Moreover, the intake of omega-3 fatty acids may eliminate chylomicrons and reduce liver production to reduce TGs and VLDL cholesterol levels (36).
This study has certain limitations that should be noted. Firstly, the average age of GDM patients included in this study was >30 years old. Although these patients belong to the high incidence of GDM age group, they are not within the general age of normal pregnancy. Thus, the included population is relatively limited, and further research is needed to confirm our conclusions. Secondly, polycystic ovary syndrome is an important risk factor for the progression of GDM itself, but because the collected clinical data was very limited, it was impossible to analyze the confounding influence of polycystic ovary syndrome on blood glucose changes.
In summary, combined supplementation with vitamin D and omega-3 fatty acids for 6 weeks in patients with GDM can effectively reduce fasting blood sugar, TGs, HDL, LDL, and total cholesterol, improve HOMA-β and insulin resistance, and ultimately effectively improve the glucose and lipid metabolism of patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist Available at http://dx.doi.org/10.21037/apm-21-1018
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-1018
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-1018). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Haikou Hospital of the Maternal and Child Health (No. 2018281) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nagao K, Yanagita T. Conjugated fatty acids in food and their health benefits. J Biosci Bioeng 2005;100:152-7. [Crossref] [PubMed]
- Scorletti E, Byrne CD. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr 2013;33:231-48. [Crossref] [PubMed]
- Chatgilialoglu C, Ferreri C, Melchiorre M, et al. Lipid geometrical isomerism: from chemistry to biology and diagnostics. Chem Rev 2014;114:255-84. [Crossref] [PubMed]
- IUPAC. Compendium of Chemical Terminology—The “Gold Book”. 2nd ed. Oxford: Blackwell Scientific Publications, 1997. (Accessed on 10 December, 2017).
- Salekzamani S, Mehralizadeh H, Ghezel A, et al. Effect of high-dose vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: a randomized controlled double-blind clinical trial. J Endocrinol Invest 2016;39:1303-13. [Crossref] [PubMed]
- Zulyniak MA, Perreault M, Gerling C, et al. Fish oil supplementation alters circulating eicosanoid concentrations in young healthy men. Metabolism 2013;62:1107-13. [Crossref] [PubMed]
- Poulos A, Sharp P, Johnson D, et al. The occurrence of polyenoic fatty acids with greater than 22 carbon atoms in mammalian spermatozoa. Biochem J 1986;240:891-5. [Crossref] [PubMed]
- Aveldaño MI, Sprecher H. Very long chain (C24 to C36) polyenoic fatty acids of the n-3 and n-6 series in dipolyunsaturated phosphatidylcholines from bovine retina. J Biol Chem 1987;262:1180-6. [Crossref] [PubMed]
- Rombeau JL, Kripke SA, Settle RG. Short-chain fatty acids production, absorption, metabolism, and intestinal effects. In: Kritchevsky D, Bonfield C, Anderson JW. editors. Dietary fiber: chemistry, physiology, and health effects. 1st ed. New York: Springer 1990:317-37.
- Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 2016;57:943-54. [Crossref] [PubMed]
- Beermann C, Jelinek J, Reinecker T, et al. Short term effects of dietary medium-chain fatty acids and n-3 long-chain polyunsaturated fatty acids on the fat metabolism of healthy volunteers. Lipids Health Dis 2003;2:10. [Crossref] [PubMed]
- Braverman N, Eichler F. Peroxisomal disorders and neurological disease. In: Squire LR. editor. Encyclopedia of neuroscience. Oxford: Academic Press, 2009:579-88.
- Sassa T, Kihara A. Metabolism of very long-chain Fatty acids: genes and pathophysiology. Biomol Ther (Seoul) 2014;22:83-92. [Crossref] [PubMed]
- Poulos A, Johnson DW, Beckman K, et al. Occurrence of unusual molecular species of sphingomyelin containing 28-34-carbon polyenoic fatty acids in ram spermatozoa. Biochem J 1987;248:961-4. [Crossref] [PubMed]
- Baidal DA, Ricordi C, Garcia-Contreras M, et al. Combination high-dose omega-3 fatty acids and high-dose cholecalciferol in new onset type 1 diabetes: a potential role in preservation of beta-cell mass. Eur Rev Med Pharmacol Sci 2016;20:3313-8. [PubMed]
- Hardy SJ, Ferrante A, Robinson BS, et al. In vitro activation of rat brain protein kinase C by polyenoic very-long-chain fatty acids. J Neurochem 1994;62:1546-51. [Crossref] [PubMed]
- Singh M. Essential fatty acids, DHA and human brain. Indian J Pediatr 2005;72:239-42. [Crossref] [PubMed]
- Arts MT, Ackman RG, Holub BJ. “Essential fatty acids” in aquatic ecosystems: a crucial link between diet and human health and evolution. Can J Fish Aquat Sci 2001;58:122-37. [Crossref]
- Osborne TB, Mendel LB. Growth on diets poor in true fats. J Biol Chem 1920;45:145-52. [Crossref]
- Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem 1929;82:345-67. [Crossref]
- Holman RT. Essential fatty acids. Nutr Rev 1958;16:33-5. [Crossref] [PubMed]
- Das UN. Essential fatty acids—biochemistry, physiology and clinical significance. In: Das UN. editor. Molecular basis of health and disease. 1st ed. Dordrecht: Springer, 2011:101-51.
- Semba RD. Essential fatty acids and visual development in infants. In: Semba RD. editor. Nutrition & health: handbook of nutrition and ophtalmology. 1st ed. Totowa: Humana Press, 2007:415-41.
- van Goor SA, Dijck-Brouwer DAJ, Muskiet FAJ. Mother-child long chain polyunsaturated fatty acid relationships: implications for diet and behavior. In: Preedy VR, Watson RR, Martin CR. editors. Handbook of behavior, food and nutrition. 1st ed. New York: Springer, 2011:1139-56.
- Parrish CC. Essential fatty acids in aquatic food webs. In: Kainz M, Brett MT, Arts MT. editors. Lipids in aquatic ecosystems. 1st ed. New York: Springer, 2009:309-26.
- Nakamura MT, Nara TY. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot Essent Fatty Acids 2003;68:145-50. [Crossref] [PubMed]
- Gurr MI, Harwood JL. Lipid biochemistry: an introduction. 4th ed. London: Chapman and Hall, 1991:406.
- Cunnane SC, Likhodii SS. 13C NMR spectroscopy and gas chromatograph--combustion--isotope ratio mass spectrometry: complementary applications in monitoring the metabolism of 13C-labelled polyunsaturated fatty acids. Can J Physiol Pharmacol 1996;74:761-8. [Crossref] [PubMed]
- Dunstan GA, Volkman JK, Jeffrey SW, et al. Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinophyceae. 2. Lipid classes and fatty acids. J Exp Mar Biol Ecol 1992;161:115-34. [Crossref]
- Gurol AO, Okten-Kursun A, Kasapoglu P, et al. The synergistic effect of omega3 and Vit D3 on glycemia and TNF-alpha in islet transplantation. Cell Mol Biol (Noisy-le-grand) 2016;62:90-8. [PubMed]
- An WS, Lee SM, Son YK, et al. Omega-3 fatty acid supplementation increases 1,25-dihydroxyvitamin D and fetuin-A levels in dialysis patients. Nutr Res 2012;32:495-502. [Crossref] [PubMed]
- Cunnane SC. The Canadian Society for Nutritional Sciences 1995 Young Scientist Award Lecture. Recent studies on the synthesis, beta-oxidation, and deficiency of linoleate and alpha-linolenate: are essential fatty acids more aptly named indispensable or conditionally dispensable fatty acids? Can J Physiol Pharmacol 1996;74:629-39. [Crossref] [PubMed]
- Davis W, Rockway S, Kwasny M. Effect of a combined therapeutic approach of intensive lipid management, omega-3 fatty acid supplementation, and increased serum 25 (OH) vitamin D on coronary calcium scores in asymptomatic adults. Am J Ther 2009;16:326-32. [Crossref] [PubMed]
- Cunnane SC. Problems with essential fatty acids: time for a new paradigm? Prog Lipid Res 2003;42:544-68. [Crossref] [PubMed]
- Algarra M, Sánchez C, Esteves da Silva JCG, et al. Fatty acid and cholestrol content of manchego type cheese prepared with incorporated avocado oil. Int J Food Prop 2012;15:796-808. [Crossref]
- Clément M, Tremblay J, Lange M, et al. Purification and identification of bovine cheese whey fatty acids exhibiting in vitro antifungal activity. J Dairy Sci 2008;91:2535-44. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


