
A systematic review and meta-analysis of effect evaluation of traditional Chinese medicine in treating acute respiratory distress syndrome
Introduction
Acute respiratory distress syndrome (ARDS) is a common clinical emergency and critical illness, with a fatality rate of about 40%. The presentation of ARDS is common in the course of serious infection, shock, trauma, and burns. It is a kind of acute diffuse inflammatory lung injury and acute respiratory failure which is caused by various pathogenic factors inside and outside the lungs caused by cardiogenic accidents (1). Patients with ARDS often present clinically with refractory hypoxemia, respiratory failure, respiratory distress, and so on. The lung imaging features ARDS patients typically show exudative lesions in both lungs. Increased pulmonary microvascular permeability, pulmonary interstitial fibrosis, exudate from the alveolar cavity, and alveolar hemorrhage are the other main pathological features of ARDS (2,3). It has been more than 50 years since ARDS was identified and established as a medical syndrome, but there is still no definite treatment for ARDS. At present, the treatment of ARDS is mostly based on mechanical ventilation, with prone position ventilation, anti-infection drugs, and organ function maintenance as auxiliary means. However, the mortality rate of patients with ARDS is still as high as 27–45% (4).
At present, the treatment of ARDS is classified into western medicine treatment and traditional Chinese medicine (TCM) treatment. Western medicine treatment is mostly based on supportive treatment, including primary disease treatment, oxygen therapy, mechanical ventilation, drug therapy, extracorporeal membrane oxygenation technology, lung transplantation, and stem cell transplantation. Although western medicine has improved the treatment of ARDS, there has been no substantial decrease in the fatality rate of ARDS patients (5). Therefore, the current western medical treatment for ARDS is still based on primary disease treatment, organ support, and mechanical ventilation. Evidence is lacking for lung transplantation, stem cell transplantation, and other non-traditional measures to support ARDS treatment, and their therapeutic effects and adverse reactions have remained unclear to date (6). Although ARDS doesn’t belong to the category of TCM diseases in China, according to the clinical characteristics of ARDS patients, including dyspnea, shallow and rapid breathing, severe suffocation, chest constriction, and so on, ARDS is classified as “severe asthma” or “asthma” in TCM. It is believed in TCM that this is caused by lung failure, lung “qi” inversion, or “qi” exchange disorder in lung and kidney (7). Therefore, TCM treatment of ARDS is mainly based on clearing away heat, detoxification, clearing the organs, promoting blood circulation, and removing blood stasis. At present, TCM treatments for ARDS include herbal compounds (Da Chengqi decoction, Xuanbai Chengqi decoction, Rhubarb aconite decoction, Ephedra aconite asarum decoction, and others), TCM injection, TCM single drug, or TCM single drug combined injection (8). A variety of therapeutic components contained in TCM can play a role in the treatment of ARDS through multiple avenues and targets (9), which have great potential for adoption in improving the clinical symptoms and reducing the mortality of ARDS patients.
However, TCM treatment of ARDS is currently still in the experimental research stage. Although there are many research reports on the treatment of ARDS with TCM, the quality of literature is inconsistent and there is a lack of uniform treatment standards. Therefore, through systematic reviews and meta-analysis, this research implemented a comprehensive evaluation of the clinical efficacy of TCM in the treatment of ARDS regarding various indicators such as case fatality rate, clinical treatment effective rate, average mechanical ventilation time, PaO2, PaCO2, PaO2/FiO2, IL-6, THF-α, and CRP. We aimed to provide a sufficient theoretical basis for the treatment of ARDS with TCM in the clinical setting. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/apm-21-1047).
Methods
Literature inclusion and exclusion criteria
The inclusion criteria were as follows. I, the research cohort were patients with a clear diagnosis of ARDS caused by various pulmonary or internal and external pathogenic factors other than cardiogenic. The gender of the patients was not limited, and their age was no less than 18 years old. II, the research type was randomized control trial (RCT) published in an English database, in the English language. III, the intervention measures of the experimental group were oral or nasal feeding TCM preparations or combined with Western medicine for conventional treatment, and the intervention measures of the control group were Western medicine conventional treatments. The baseline data of the experimental group and the control group were comparable.
The exclusion criteria were as follows. (I) Research types were retrospective studies, cohort studies, case reports, and other non-RCT studies. (II) The research cohort was non-ARDS, or the research participants were animals, children, or cells. (III) The treatment method of the literature experimental group was not TCM treatment or combined TCM and Western medicine treatment. (IV) Unpublished literature or non-English literature such as degree thesis. (V) Trials involving ARDS patients combined with other diseases. (VI) Literature with incomplete research data or literature published in duplicate.
Literature retrieval
We searched a total of 6 English language databases (PubMed, Embase, Medline, Spring, Cochrane Library, and Web of Sciences). The search type was RCT research on ARDS published from 1 January 2001 to 31 December 2020. The literature search terms consisted of subject words and free words, including “Acute Respiratory Distress Syndrome”, “Traditional Chinese Medicine”, “randomized controlled trial”, “ALI”, and “Chinese herbal medicine”. The search terms were searched together with “and” or “or”, and the literature search was carried out by 2 research institutes using independent back-to-back searches.
Literature screening
The articles retrieved from the search were screened by 2 evaluators independently. After retrieval, the literature title was imported into the Note Express 3.2 (http://www.inoteexpress.com/aegean/) literature manager to establish a literature database. Then, Note Express 3.2 software was used to check for and eliminate duplicate literature. After the preliminary screening was complete, the assessor Wen Liang continued with manual screening, firstly by reading the title and abstract of the retrieved literature. Literature that obviously did not meet the inclusion criteria was eliminated. Then, the full text was read carefully with respect to the inclusion and exclusion criteria to determine whether it would be included. If there were disagreements, the 2 assessors would reach consensus via discussion. If consensus was still not reached, a decision was made by a third party.
Data extraction
The 2 evaluators independently utilized self-developed data extraction forms to extract data that met the inclusion criteria, which were then cross-checked after extraction. The literature extraction materials that met the inclusion criteria included the following: (I) literature title, first author (only 1 name included), publication year, and journal; (II) research participants’ age, gender, sample size, and baseline comparability; (III) intervention measures and control measures; (IV) outcome indicators, including case fatality rate, effective rate of clinical treatment, mechanical ventilation time, length of stay in intensive care unit (ICU), and respiratory mechanics parameters. If there were disagreements, the 2 assessors entered discussion to reach consensus. If consensus was not reached, the final decision was made by a third party.
Evaluation of literature bias risk
A risk of bias assessment was made according to the risk of bias assessment standard provided by the Cochrane Handbook version 5.0.2 of the systematic review manual. The evaluation criteria included selection bias, implementation bias, measurement bias, and follow-up bias. Specifically, it included whether it was a random sequence, if hiding was assigned, if the subjects were blinded, whether the outcome assessor was blinded, whether the data was complete, if there was selective reporting, and whether there were other biases present. If the 2 assessors disagreed with each other, they negotiated between themselves or a third party was used to make a ruling.
Statistical processing
The bias analysis tool in Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (https://handbook-5-1.cochrane.org) was employed to evaluate the risk of literature bias. The software STATA 12.0 (https://www.stata.com) was adopted to organize the data of the included literature. Review Manager 5.3 software (RevMan, The Cochrane Collaboration, http://tech.cochrane.org/revman) was employed for meta-analysis. If the unit of measurement was not uniform between different samples, it was expressed in the form of standardized mean difference (SMD). If it was found that the results of each study could be combined, meta-analysis was performed. The combined effect size test adopted u-test and 95% confidence interval (CI). The u-test result was expressed as a P value. When P<0.05, the difference was considered statistically significant. Binary variables adopted a relative risk (RR) and 95% CI as effect size, and continuous variables took SMD and 95% CI as effect size. According to the heterogeneity (I2), fixed effects model (FEM) or random effects models (REM) was selected to combine effect size. Sensitivity analysis was implemented to test whether the meta-analysis results were stable and explore the source of heterogeneity. If the possible sources of heterogeneity were found, FEM was adopted for meta-analysis. If the source of heterogeneity was not found, REM was adopted for meta-analysis. If meta-analysis was not possible (research data was less than 2), descriptive analysis was performed. In the final analysis, P<0.05 indicated a statistical difference.
Results
Literature search results
Corresponding keywords were entered in 6 English language databases for preliminary retrieval of materials, and a total of 1,428 related studies were retrieved. Among these, 429 were from PubMed, 338 from Embase, 361 from Medline, 205 from Spring, 188 from Cochrane Library, and 268 were from Web of Sciences. After the preliminary search was completed, Endnote X8 software (https://endnote.com) was adopted to eliminate duplicate literature, and 1,022 studies were left. Then, 2 literature evaluators read the literature titles and abstracts, and excluded 899 studies that obviously did not meet the inclusion criteria. The remaining 123 studies were carefully read and cross-checked by 2 evaluators, and studies that did not meet the inclusion and exclusion criteria were eliminated. Finally, a total of 16 studies were included in this work (Figure 1, Table 1). The 16 included studies contained 1,460 research participants, including 731 in the experimental group and 729 in the control group.

Full table
Bias risk assessment of included literature
The Cochrane Handbook 5.0.2 bias risk assessment tool was employed to evaluate the bias risk of all literatures included in this meta-analysis. The evaluation results were input into Review Manager 5.3 software to generate a bias risk map. (I) Random sequence generation: the 16 papers included in this analysis all reported the adoption of random grouping. Among which, 9 studies (11,13,17,19-21,23-25) described specific random methods, indicating low risk. The other 7 studies (10,12,14-16,18,22) only mentioned random grouping, and did not inform the specific random method, suggesting unclear risk. (II) Assignment blinding: a single study clearly mentioned that the grouping method was single blind (18), indicating low risk, and the other 15 studies (10-17,19-25) did not show whether they adopted blinding, indicating unclear risk. (III) Participant blinding: 9 papers (14-19,21,23,24) clearly mentioned “patients signed the informed consent forms”, but did not mention whether they blinded the test personnel, suggesting high risk. The other 7 studies (10-13,20,22,25) did mention whether participants were blinded, suggesting unclear risk. (IV) Blind method of outcome evaluator: all 16 papers reported whether the outcome evaluator was blinded, indicating unclear risk. (V) Data integrity: the outcome data of all 16 papers were complete, indicating low risk. (VI) Selective report: 3 papers (13,14,20) could not be judged as selective reports, suggesting unclear risk, and the other 13 studies (10-12,15-19,21-25) had no selective report, suggesting low risk. (VII) Other biases: there were 3 studies (10,11,21) that included inconsistent numbers of participants in the experimental and the control groups, indicating high risk. The other 13 studies (12-20,22-25) were uncertain whether there were other biases, suggesting unclear risk. The detailed results of this bias analysis are shown in Figures 2,3.
Case fatality rate
A total of 6 of the 16 studies included reported on the mortality rate of ARDS treated by TCM (10,11,13,16-18). A total of 563 participants were included, with 284 in the observation group and 279 in the control group. After statistical analysis, it was found that the heterogeneity between the observation group and the control group was small (I2=0%, P=0.52). Therefore, the fixed effects model was adopted for analysis. As shown in Figure 4, the combined effect of meta-analysis was (MD =0.51; 95% CI: 0.32–0.84; Z=2.69; P=0.007), suggesting that the TCM treatment group had a lower-case fatality rate than the control group. There was a considerable difference in mortality between the treatment group and the control group. Thus, it was suggested that compared with the conventional treatment of western medicine, the treatment of ARDS with TCM can remarkably reduce the mortality of patients.
Efficiency of clinical treatment
Among the 16 papers included in this study, a total of 7 reported on the effective rate of the clinical treatment of ARDS by TCM (12,13,18-20,22,25). A total of 815 participants were included, with 415 in the observation group and 400 in the control group. After statistical analysis, it was found that the heterogeneity between the observation group and the control group was small (I2=0%, P=0.55), so the fixed effects model was adopted for analysis. As shown in Figure 5, the combined effect of meta-analysis was (MD =2.64; 95% CI: 1.79–3.90; Z=4.90; P<0.00001), suggesting that with the treatment of ARDS with TCM, the observation group had a considerable difference in clinical treatment efficiency compared with the control group. It was shown that the curative effect of integrated TCM and western medicine in the treatment of ARDS is better than that of conventional western medicine alone
Mean mechanical ventilation time
A total of 8 of the 16 literatures included in this study reported on the average mechanical ventilation time of TCM treatment of ARDS (13-18,24,25). A total of 794 participants were included, including 397 in the observation group and 397 in the control group. After statistical analysis, it was found that there was heterogeneity between the observation group and the control group (I2=94%, P<0.00001), so the random effects model was adopted for analysis. As shown in Figure 6, the combined effect of meta-analysis was (MD =−3.14; 95% CI: −4.07 to −2.21; Z=6.64; P<0.00001), indicating that in the treatment of ARDS with TCM, the observation group had a considerable difference in the average mechanical ventilation time compared with the control group. It is suggested that TCM treatment of ARDS can reduce the average mechanical ventilation time and promote the recovery of respiratory function.
PaO2
A total of 9 of the 16 papers included in this study reported on the PaO2 in the treatment of ARDS with TCM (10,12,18-24). A total of 831 participants were included, with 418 in the observation group and 413 in the control group. After statistics, it was found that there was heterogeneity between the observation group and the control group (I2=91%, P<0.00001), so the random effects model was adopted for analysis. As shown in Figure 7, the combined effect of meta-analysis was (MD =12.29; 95% CI: 8.88–15.71; Z=7.05; P<0.00001), indicating that there was a considerable difference in PaO2 between the observation group and the control group after treatment of ARDS with TCM. It was verified that TCM can remarkably increase the PaO2 level of patients after ARDS.
PaCO2
A total of 7 of the 16 studies reported on the PaCO2 in patients with ARDS treated with TCM (12,18-22,24). A total of 744 participants were included, with 374 in the observation group and 370 in the control group. After statistics, it was found that there was heterogeneity between the observation group and the control group (I2=91%, P<0.00001), so the random effects model was adopted for analysis. As shown in Figure 8, the combined effect of meta-analysis was (MD =−0.16; 95% CI: −2.97–2.65; Z=0.11; P=0.91), indicating that there was no statistical difference in PaCO2 levels between the observation group and the control group after ARDS treatment with TCM.
PaO2/FiO2
A total of 7 of the 16 literatures included in this study reported on the oxygenation index (PaO2/FiO2) of ARDS patients treated with TCM (13,14,16,18,21,23,24). A total of 632 participants were included, with 318 in the observation group and 314 in the control group. After statistical analysis, heterogeneity was detected between the observation group and the control group (I2=85%, P<0.00001), so the random effects model was adopted for analysis. As shown in Figure 9, the combined effect of meta-analysis was (MD =50.70; 95% CI: 32.78–68.63; Z=5.54; P<0.00001), indicating that there was a considerable difference in PaO2/FiO2 between the observation group and the control group after ARDS treatment with TCM. This suggested that TCM treatment of ARDS has more advantages than conventional western medicine treatment alone in improving PaO2/FiO2.
Interleukin 6 (IL-6)
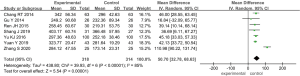
A total of 7 of the 16 studies included reported on IL-6 in patients with ARDS treated with TCM (11,17,18,21-23,25). A total of 806 participants were included, with 406 in the observation group and 400 in the control group. After statistical analysis, it was found that there was heterogeneity between the observation group and the control group (I2=97%, P<0.00001), so the random effects model was adopted for analysis. As shown in Figure 10, the combined effect of meta-analysis was (MD =−8.32; 95% CI: −11.48 to −5.17; Z=5.17; P<0.00001), indicating that there was a considerable difference in serum IL-6 levels between the observation group and the control group after ARDS treatment with TCM. It was suggested that TCM treatment of ARDS has more advantages than conventional western medicine treatment in reducing the levels of IL-6.
Tumor necrosis factor alpha (TNF-α)
Among the 16 studies included in this meta-analysis, a total of 7 reported on TNF-α after treatment with TCM in patients with ARDS (11,17-19,21,23,25). A total of 650 participants were included, with 328 in the observation group and 322 in the control group. After statistical analysis, heterogeneity was detected between the observation group and the control group (I2=98%, P<0.00001), so the random effects model was adopted for analysis. As shown in Figure 11, the combined effect of meta-analysis was (MD =−11.22; 95% CI: −17.14 to −5.31; Z=3.72; P=0.0002), indicating that there was considerable difference in the level of TNF-α between the observation group and the control group after ARDS treatment with TCM. This suggested that compared with the conventional treatment of western medicine, the treatment of ARDS with TCM can more remarkably reduce the level of TNF-α.
C-reactive protein
A total of 6 of the 16 studies included in this meta-analysis reported on C-reactive protein (CRP) in patients with ARDS treated with TCM (16-18,21,22,25). A total of 722 participants were included, with 363 cases in the observation group and 359 cases in the control group. After statistical analysis, heterogeneity was detected between the observation group and the control group (I2=95%, P<0.00001), so the random effects model was adopted for analysis. As shown in Figure 12, the combined result of the meta-analysis effect value was (MD =−9.23; 95% CI: −14.23 to −4.24; Z=3.62; P=0.0003), which indicated that there was considerable difference in CRP level between the observation group and the control group after ARDS treatment with TCM. This suggested that compared with the conventional treatment of western medicine, the treatment of ARDS with TCM can more remarkably reduce the level of CRP.
Publication bias analysis
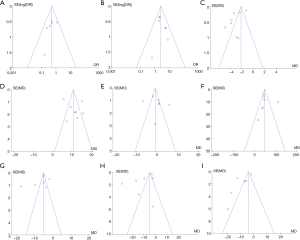
Review Manager 5.3 is employed to conduct publication bias analysis on the analysis indicators of the efficacy of TCM treatment of ARDS, and the results are presented in Figure 13. Patient mortality, clinical treatment effective rate, PaCO2 level, and PaO2/FiO2 level were basically distributed within the CI, and literature bias was small. In the funnel plot of patients’ average mechanical ventilation time, PaO2 level, IL-6, TNF-α, and CRP, the distribution of the points representing literature is relatively scattered, and some of the scattered points fall outside the CI, suggesting that the included literatures were biased.
Discussion
The presence of ARDS is the initiating factor of multiple organ failure in the human body. At present, clinical treatment is still based on the treatment of the original disease combined with the treatment of mechanical ventilation, and the prognosis is generally poor. The clinical symptoms of ARDS, such as dyspnea, rapid shallow breathing, tight chest, and others, coincide with TCM’s categories of “violent asthma” and “uncontrolled asthma”. Therefore, TCM treatment to improve the survival of patients with ARDS has important research value. At present, many clinical studies on the treatment of ARDS with integrated TCM and western medicine have shown that after treatment with TCM, the survival rate of ARDS patients was remarkably improved. In addition, ARDS can improve clinical treatment efficiency and reduce mechanical ventilation time (26). The levels of PaO2, PaCO2, and PaO2/FiO2 have important value in the diagnosis of hypoxemia and respiratory failure. The marker IL-6 is a kind of lymphokine produced by T cells or fibroblasts that can stimulate the activation of B cells. The marker THF-α is an inflammatory mediator that can assess oxidative stress and inflammation in the body. The marker CRP is an acute stress protein that is secreted when the body encounters microbial invasion or tissue damage and participates in the body’s non-specific immunity (27). In the treatment of ARDS, PaO2, PaCO2, PaO2/FiO2, IL-6, TNF-α, CRP, and so on, have a certain effect of promotion or inhibition.
In recent years, there have also been some other meta-analyses on the treatment of ARDS with TCM. Wang (28) asserted that TCM can reduce the mortality rate of patients with ARDS, improve the effective rate of clinical treatment, reduce the average mechanical ventilation time, improve PaO2/FiO2, and lower IL-6 levels. The meta-analysis by Chen et al. (29) found that the TCM method of purging the lungs and organs can reduce the mortality rate, shorten the time of mechanical ventilation, and improve PaO2/FiO2. However, different scholars have followed different research directions on the treatment of ARDS with TCM, and there are also certain differences in research directions and research results. To address the irregularities of literature on the treatment of ARDS with TCM, and to fill in the gaps identified in previous studies, we conducted a systematic evaluation of the treatment of ARDS by TCM through meta-analysis, and analyzed the therapeutic effect of TCM on ARDS patients.
Conclusions
A total of 16 studies were included in this meta-analysis on TCM treatment of ARDS. A total of 1,460 ARDS patients were involved in this study. Treatment methods included Da Chengqi decoction, Modified Xuanbai Chengqi decoction, Ephedra aconite Asarum decoction, Qingfei decoction, Baihu Chengqi decoction, Tingli Dazao Xiefei decoction, Xiao Qinglong decoction, and Fusu mixture. The results of this meta-analysis showed that compared with conventional Western medicine treatment, TCM treatment of ARDS can remarkably reduce patient mortality. It can improve the clinical treatment efficiency, reduce the average mechanical ventilation time, and improve the levels of PaO2 and PaO2/FiO2. Moreover, IL-6, TNF-α, and CRP levels were decreased, but PaCO2 levels were not remarkably affected.
This work also had certain limitations, which were manifested in the fact that it was not clear whether some studies used the blind method, which led to a greater bias in some literatures. In addition, due to the different research directions of authors, some indicators covered a small number of samples, and meta-analysis could not be performed or the analysis results were not accurate enough. Therefore, in future work, it is still necessary to select more high-quality, large-sample RCTs to provide more systematic support for the treatment of ARDS with TCM.
Acknowledgments
Funding: This study was supported by grants from the Yichang Key Laboratory of the Preservation and Innovation for Chinese Medicine Proved Recipe, China (443002) and the Yichang Science and Technology Bureau Special Fund (A20-2-038) and 2020 Research Project on Graduate Teaching Reform of Three Gorges University (SDYJ202022).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-1047
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-1047). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shah RD, Wunderink RG. Viral Pneumonia and Acute Respiratory Distress Syndrome. Clin Chest Med 2017;38:113-25. [Crossref] [PubMed]
- Yadav H, Thompson BT, Gajic O. Fifty Years of Research in ARDS. Is Acute Respiratory Distress Syndrome a Preventable Disease? Am J Respir Crit Care Med 2017;195:725-36. [Crossref] [PubMed]
- Derwall M, Martin L, Rossaint R. The acute respiratory distress syndrome: pathophysiology, current clinical practice, and emerging therapies. Expert Rev Respir Med 2018;12:1021-9. [Crossref] [PubMed]
- Shaw TD, McAuley DF, O'Kane CM. Emerging drugs for treating the acute respiratory distress syndrome. Expert Opin Emerg Drugs 2019;24:29-41. [Crossref] [PubMed]
- Virani A, Ma K, Leap J, et al. Acute Respiratory Distress Syndrome Definition, Causes, and Pathophysiology. Crit Care Nurs Q 2019;42:344-8. [Crossref] [PubMed]
- Fu S, Thangavel S, Ivanova V. Cardiac Dysfunction in Acute Respiratory Distress Syndrome. Crit Care Nurs Q 2019;42:448-58. [Crossref] [PubMed]
- Zhang S, Zhang L, Long KL, et al. Evaluation of clinical efficacy of integrated traditional Chinese and Western medicine in the treatment of acute respiratory distress syndrome. Medicine (Baltimore) 2020;99:e20341 [Crossref] [PubMed]
- Liu Y, Zhang XQ, Dong Y, et al. Research progress of traditional Chinese medicine in treatment of acute respiratory distress syndrome. Hebei Medical Journal 2020;42:298-302.
- Duan Z, Kong L, Hao H, et al. Discussion on Traditional Chinese Medicine Pathogenesis of Acute Respiratory Distress Syndrome. Journal of Emergency in Traditional Chinese Medicine 1992;2020:1979-1980.
- Zhong KL, Tian D, Huang Y. Observation of therapeutic effects of Dachengqi decoction combined with mechanical ventilation on patients with acute respiratory distress syndrome. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care 2006;13:288-90.
- Xie XH, Yang YF, Zhang XQ, et al. Effect of Tongfu Xiere Recipe on Cytokine Level in Patients with Acute Lung Injury Due to Surgery Disease of Chest. Journal of Guangzhou University of Traditional Chinese Medicine 2008;25:294-7.
- Tuo CD, Guo SM, Liu Y. To Observa Curative Effect of Enema of Xuanbaichengqi Decoction on Acute Lung Injury. Journal of Emergency in Traditional Chinese Medicine 2013;22:1128, 1131.
- Chang RT, Shang DM, Jiang JY. Curative Observation on MaHuang FuZi XiXin Decoction in Treating Acute Respiratory Distress Syndrome. Western Journal of Traditional Chinese Medicine 2014;27:99-101.
- Gu Y, Chen JR, Shao F, et al. Effects of Qingfeitang on ET-1 and PGE2 in exhaled breath condensate and serum of patients with ARDS. The Journal of Practical Medicine 2014;30:3853-5.
- Chen ZP, Liu XF. Clinical Study on the Treatment of Acute Lung Injury with Mechanical Ventilation by Xuanbai Chengqi Tang. Journal of Henan University of Chinese Medicine 2015;30:1104-5.
- Ren JH, Chen JR, Gao X, et al. Clinical Observation on the Effects of Qingfei Tang in Treating ARDS and the Influence on ET-1 and 8-iso-PG Levels. Journal of Nanjing University of Traditional Chinese Medicine 2015;31:323-6. (Natural Science).
- Liu HW, Tang JW, Rao LL. Application value of Dachengqi decoction for the patients with acute lung injury/acute respiratory distress syndrome in mechanical ventilation. Modern Journal of Integrated Chinese Traditional and Western Medicine 2016;25:3792-3794, 3876.
- Yu KJ, Qiu ZX. Clinical Observation on Effect of Tingli Dazao Xiefei Decoction in Treating Pulmonary Contusion with Acute Respiratory Distress Syndrome. Shijie Zhongyiyao 2016;11:1711-3.
- Feng B, Mao ZR, Deng Y. Regulative effect of Xuanbai Chengqi Decoction on TNF-α and IL-1β in patients with acute respiratory distress syndrome and its mechanical indexes. The Journal of Practical Medicine 2017;33:1337-40.
- Ma JQ, Liu P. Observation on the Curative Effect of Jiawei Xuan Cheng Qi Decoction and Western Medicine in the Treatment of Acute Respiratory Distress Syndrome. China Reflexology 2018;27:6-8.
- Shang J, Bai FY, Zheng JQ. The clinical effect of the Xuanbai Chengqi decoction on acute lung injury/acute respiratory distress syndrome. Clinical Journal of Chinese Medicine 2019;11:6-9.
- Wang M, Yan L. Study on the efficacy and mechanism of Baihu Chengqi decoction in the treatment of severe pneumonia. Shaanxi Journal of Traditional Chinese Medicine 2019;40:1693-6.
- Yuan Y. Clinical Observation on Treatment of 80 Cases of Acute Respiratory Distress Syndrome with Xiaoqinglong Decoction. Chinese Primary Health Care 2019;33:80-82, 90.
- Zhang S, Ding P, Xu MX, et al. Effect of Fusu Mixture on mechanical ventilation in patients with acute re-spiratory distress syndrome. Journal of North Sichuan Medical College 2020;35:220-3.
- Yang JL, Wang YZ, Guo XY, et al. Effect of Xuanfei Shenshi decoction combined with acupoint application on acute respiratory distress syndrome in adults. Shaanxi Journal of Traditional Chinese Medicine 2020;41:312-4.
- Zhang WB, Jiang Y, Liu XW, et al. Investigation of mechanical ventilation pressure parameters' effect on the prognosis of acute respiratory distress syndrome. Chinese Journal of Emergency Medicine 2020;29:121-5.
- Duan YN, Mi T. Changes of serum IL-18 and tissue factor levels in patients with acute respiratory distress syndrome and their relationships with prognosis. Journal of Critical Care In Internal Medicine 2020;26:119-121, 137.
- Wang MT, Li Y, Liang X, et al. Meta-analysis of clinical efficacy of integrated Chinese traditional and western medicine therapy in the treatment of acute respiratory distress syndrome. Global Chinese Medicine 2018;11:315-20.
- Cheng L, Zhang Y, He SY, et al. A systematic review and Meta-analysis of Tongfu Xiefei method in the treatment of acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020;32:970-5. [PubMed]
(English Language Editor: J. Jones)













