
A clinical study of lyophilized intravenous human immunoglobulin containing high-titer cytomegalovirus-neutralizing antibody for the treatment of cytomegalovirus viremia after allogeneic hematopoietic stem cell transplantation
Introduction
Cytomegalovirus (CMV) infection is a common complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT). It may manifest as simple CMV viremia, CMV syndrome with fever as the main symptom, and, in serious cases, CMV diseases (such as pneumonia, hepatitis, gastrointestinal disorders, retinitis, encephalitis) (1). CMV infection also increases the risk of graft versus host disease (GVHD) and poor graft function, leading to graft failure (2). Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) requires more intense immunosuppression, which increases the risk of CMV viremia and associated complications. At present, ganciclovir (GCV) and foscarnet sodium are main treatments for post-allo-HSCT CMV viremia, but they are ineffective in some patients (3,4). Therefore, it is important to look for more effective and safer anti-CMV drugs.
Lyophilized intravenous human immunoglobulin containing high-titer CMV-neutralizing antibody (CMV-IVIG) is a specific anti-CMV human immunoglobulin. Foreign researchers have used CMV-IVIG as adjuvant treatment for post-HSCT CMV infection and have found that the combination of CMV-IVIG and antiviral drugs may further improve treatment outcomes. However, CMV-IVIG has not been widely used in China to treat post-allo-HSCT CMV viremia, and no related reports are available. This study retrospectively evaluated the efficacy of concurrent CMV-IVIG at different time points for the treatment of CMV viremia at our hospital.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-1069).
Methods
Subjects
The protocol was approved by the Ethics Committee, Institute of Hematology & Hospital of Blood Diseases, Chinese Academy of Medical Sciences (HG2021009-EC-1). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. The clinical data of patients who received allo-HSCT and concurrent CMV-IVIG (Chengdu Rongsheng Pharmaceutical Co., Ltd., Chengdu, China) to treat post-allo-HSCT CMV viremia at our hospital between January and September 2020 were collected. The patients were divided into two groups based on the total dose of antithymocyte globulin (ATG) used in the pretransplant preconditioning regimen, i.e., the low-dose ATG group (rabbit ATG ≤6 mg/kg, porcine ATG ≤40 mg/kg) and the high-dose ATG group (rabbit ATG >6 mg/kg, porcine ATG >40 mg/kg).
Pretransplant preconditioning regimen
Patients with severe aplastic anemia (SAA) received ATG + fludarabine (Flu) + cyclophosphamide (Cy); patients with acute/chronic myelogenous leukemia (AML/CML), myelodysplastic syndrome (MDS), chronic myelomonocytic leukemia (CMML), or myeloproliferative neoplasms (MPN) received busulfan (Bu) + Cy + Flu + cytarabine (Ara-C); and patients with acute lymphoblastic leukemia (ALL) or lymphoma received total body irradiation (TBI) + Cy + Flu + Ara-C; some patients also received idarubicin (IDA), etoposide (VP-16), cladribine, or decitabine (DAC).
GVHD prevention and treatment
To prevent GVHD, patients with a human leukocyte antigen (HLA)-identical sibling donor received cyclosporine A or tacrolimus plus a short course of methotrexate; patients with an HLA-haploidentical donor received cyclosporine A or tacrolimus plus mycophenolate mofetil. When GVHD occurred, corticosteroids were the first-line treatment, and basiliximab or rucotinib were used if corticosteroid was ineffective.
GVHD was classified according to the diagnostic criteria for acute GVHD (aGVHD) (5) and chronic GVHD (cGVHD) (6).
Monitoring of CMV infection
Real-time quantitative polymerase chain reaction (RQ-PCR) was performed to monitor CMV-DNA levels in peripheral blood, urine, and stool once or twice a week for 3 to 6 months after transplantation (infusion of hematopoietic stem cells).
Diagnosis of CMV viremia and CMV diseases
CMV viremia: CMV-DNA >1,000 copies/mL in blood (RQ-PCR). CMV diseases: CMV-DNA >1,000 copies/mL in blood, urine, or stool plus clinical manifestations, or positive CMV from target organ or tissue.
Treatment of CMV infection
Upon the diagnosis of CMV viremia, anti-CMV therapy (induction therapy with GCV 5 mg/kg q12h or foscarnet sodium 90 mg/kg q12h for 1–2 weeks, followed by maintenance therapy) was initiated. CMV-IVIG may not be given or may be given early (within 3 days after the diagnosis of CMV viremia) or late (after 3 days) depending on individual conditions and economic factors. CMV-IVIG was intravenously infused at 100 mg/kg once every two days, and an integral number of vials was used whenever possible. After CMV-DNA conversion (confirmed with two consecutive tests), CMV-IVIG was administered once a week for two weeks.
Statistical analysis
SPSS (SPSS, Chicago IL, USA) was used for statistical analysis. The Kolmogorov-Smirnov test was performed to evaluate the normality of continuous variables. Normally distributed variables were analyzed with the t-test, and nonnormally distributed variables were analyzed with the Mann-Whitney U test. The Kaplan-Meier curve was used to analyze CMV-DNA conversion over time, and the log-rank test was performed for intergroup comparisons. All tests were two-sided, and P<0.05 was considered statistically significant.
Results
CMV viremia under different CMV-IVIG treatment strategies
A total of 73 patients received CMV-IVIG combined with antiviral therapy to treat post-allo-HSCT CMV viremia. Table 1 shows CMV-IVIG treatment strategy and patient characteristics. Two patients received early CMV-IVIG therapy but discontinued CMV-IVIG for reasons unrelated to CMV infection, and one patient who received early CMV-IVIG therapy died 4 days later for reasons unrelated to CMV infection. The remaining 70 patients (early CMV-IVIG therapy group: n =27, late CMV-IVIG therapy group: n=43) received standard anti-CMV therapy, with no deaths during the period of this study.
Full table
Table 2 shows the information for CMV infection in 73 patients with CMV viremia. No significant difference was observed in the initial CMV-DNA load at the first positive CMV-DNA test between the early CMV-IVIG therapy group and the late CMV-IVIG therapy group, but the median maximum CMV-DNA load was significantly lower in the early CMV-IVIG therapy group (P=0.001). Moreover, the incidence of CMV diseases was significantly lower in the early CMV-IVIG therapy group; only 2 patients had CMV enteritis before CMV-IVIG therapy, and there were no new cases after CMV-IVIG therapy. In the late CMV-IVIG therapy group, none of the patients received CMV-IVIG at the onset of CMV disease except for one patient with CMV pneumonia. No CMV disease-related deaths occurred in either group. These data indicate that concurrent CMV-IVIG therapy may reduce the incidence of CMV diseases.

Full table
Treatment outcomes for early versus late CMV-IVIG therapy
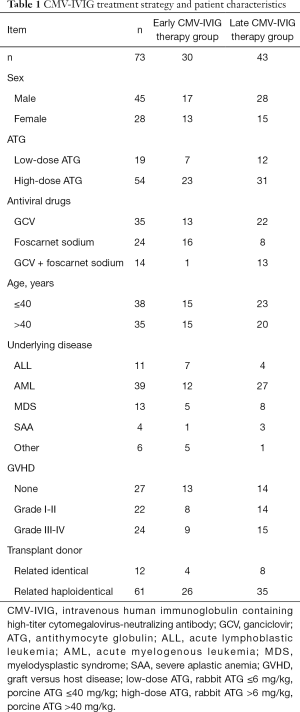
The overall response rate for CMV-IVIG combined with traditional anti-CMV drugs was 100%. In the early CMV-IVIG therapy group (n=27), the CMV-DNA conversion rate was 100%, the two-week response rate was 62.96% (17/27), and the median time from treatment to CMV clearance was 14 days. In the late CMV-IVIG therapy group (n=43), the CMV-DNA conversion rate was 100%, the two-week response rate was 16.28% (7/43), and the median time of conversion was 21 days, which was significantly longer than that in the early CMV-IVIG therapy group (P=0.000, Figure 1).
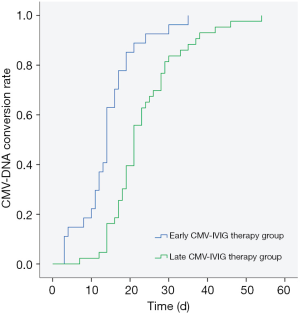
In the low-dose ATG group, the two-week response rates were 66.67% (4/6) in the early CMV-IVIG therapy group and 25.00% (3/12) in the late CMV-IVIG therapy group, and the median times of CMV-DNA conversion were 11 and 18 days, respectively (P=0.291). In the high-dose ATG group, the two-week response rates were 61.90% (13/21) in the early CMV-IVIG therapy group and 12.90% (4/31) in the late CMV-IVIG therapy group, and the median times of CMV-DNA conversion were 14 and 23 days, respectively (P=0.000) (Figure 2). These data indicate that for patients with post-allo-HSCT CMV viremia, early CMV-IVIG therapy can effectively accelerate CMV-DNA conversion and peripheral CMV clearance and significantly improve the two-week response rate. The effects were more pronounced in patients receiving high-dose ATG.

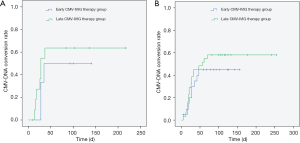
Regarding reactivation, CMV-DNA remained negative for more than 60 days in 45.21% of the patients and for more than 100 days in 31.51% of the patients. In the low-dose ATG group, the reactivation rate was 50.00% in patients who received early CMV-IVIG therapy and 63.64% in patients who received late CMV-IVIG therapy; and in the high-dose ATG group, the numbers were 45.00% and 58.06%, respectively. While the differences were not statistically significant, early CMV-IVIG therapy showed a tendency to reduce the reactivation rate (Figure 3).
Adverse reactions
Among the patients treated with GCV or foscarnet sodium, 5 experienced bone marrow suppression with different degrees of severity, which mainly manifested as relapsed neutropenia and/or thrombocytopenia. One patient who received foscarnet sodium experienced fever, which was considered to be related to antiviral therapy; foscarnet sodium was discontinued, and the symptom improved after conversion to GCV. CMV-IVIG was well tolerated, and no major adverse drug reactions were observed.
Discussion
Allo-HSCT can cause marked immunosuppression, which interferes with T-cell reconstruction after transplantation. The immune reconstruction process is correlated strongly with different kinds of opportunistic infections. Prior to reconstruction of adaptive immunity, there is an increased susceptibility to viral infection, such as herpes group virus. CMV infection is a common cause of early posttransplant mortality unrelated to the primary disease. Previous known risk factors of CMV reactivation were CMV serostatus, transplantation type, T-cell depletion regimen, and GVHD development. In clinical practice, identifying patients at high risk for the development of CMV disease and patient characteristics associated with a high mortality rate may be more relevant for optimizing their treatment.
Preemptive treatment strategies based on CMV-DNA testing have been widely used across transplant centers. At present, intravenous GCV or foscarnet sodium is the standard treatment for posttransplant CMV infection; however, some patients do not respond to the treatment. Reusser et al. (7) conducted a randomized study and showed that the event-free survival rates were 73% for preemptive GCV treatment and 66% for preemptive foscarnet treatment of CMV infection, suggesting that drug therapy alone is not sufficient to completely suppress CMV-DNA replication. At our hospital, anti-CMV therapy should be administered once CMV-DNA is >1,000 copies/mL in peripheral blood. This study showed that 98.63% of CMV viremia cases occurred within 100 days after transplantation, and the overall CMV-DNA conversion rate was 100% in 70 patients with CMV viremia after preemptive treatment that combined CMV-IVIG with GCV or foscarnet sodium.
CMV infection results in the development of an adaptive immune response involving humoral and cellular factors. The relative importance of humoral immunity in humans is not clear. Some researches indicated that the presence of high titer antibodies has been associated with protection from CMV reactivation (8-10). Zdziarski (11) demonstrated that before primary cellular immune response development, the high level of residual CMV-IgG (about >100 R/mL) from HSCT recipient prevents virus reactivation, meanwhile, CMV-IgG significant decrease predicts CMV replication. Generally, the antibody produced from recent infection has strong affinity.
Immunotherapy remains an area of active investigation for the treatment and prophylaxis of CMV infections. MSL-109 is a human monoclonal IgG originally isolated from spleen cells of a CMV seropositive individual. However, MSL-109 did not demonstrate benefit in all-comers. Like other herpesviruses, CMV uses multiprotein entry complexes to initiate infection of host cells. Actually, a single epitope antibody cannot completely prevent the virus from invading the sensitive cells. CMV-IVIG is a specific anti-CMV immunoglobulin preparation. Compared with regular human immunoglobulins, CMV-IVIG contains high-titer CMV-neutralizing antibody (≥600 U/mL), which specifically binds to neutralizing antigens, such as CMV envelope glycoprotein gB/gN/gM, to block antigen epitopes and prevent the virus from fusing with the cell membrane. This stops the virus from entering the host cell, thereby interfering with viral replication. In addition, CMV-IVIG is rich in IgG1 and IgG3, which are potent for complement activation and the induction of the complement cascade. Moreover, IgG antibody binds to CMV and induces Fc receptor-mediated phagocytosis to clear CMV. Intravenously administered CMV-IVIG is fast acting with few allergic reactions and has the potential for broad clinical application.
Several studies have shown that CMV-IVIG is effective for the prevention and treatment of CMV infection in recipients of solid organ and stem cell transplantation (12-17). A retrospective study in the US analyzed 35 cases of CMV diseases (CMV pneumonia: n=26; CMV enteritis: n=9) after HSCT (14), and the results showed that CMV-IVIG (150 mg/kg, 2–3 times per week) combined with anti-CMV drugs reduced mortality compared to treatment with anti-CMV drugs alone. In this study, the treatment outcomes of 70 patients with CMV infection were analyzed. The results of 27 patients who received early CMV-IVIG therapy combined with traditional anti-CMV treatment were compared to those of patients who received late CMV-IVIG therapy. In those who received early treatment, the median time of CMV-DNA conversion was reduced by 7 days, and the two-week response rate was improved by 46.68%; therefore, the treatment time was significantly reduced. Moreover, unlike traditional anti-CMV drugs, CMV-IVIG did not induce major adverse reactions, such as bone marrow suppression.
With the advancement of transplantation technology, the rate of related haploidentical donor transplantation is increasing. In this study, 61 patients received related haploidentical donor transplantation, and 12 received related identical donor transplantation. The incidence of GVHD is higher with haploidentical transplantation, which necessitates the use of ATG. While ATG reduce the incidence of GVHD, its long half-life may cause delayed immune reconstitution, particularly delayed cluster of differentiation (CD)4+ T cell reconstitution, which increases the risk of CMV infection (18). This study showed that in the high-dose ATG group, patients with CMV infection who received early CMV-IVIG therapy combined with traditional anti-CMV drugs had a median time of CMV-DNA conversion of only 14 days, a two-week response rate of 61.90%, and a lower reactivation rate than patients who received late CMV-IVIG therapy combined with traditional anti-CMV drugs.
There are limitations to the present study that should be highlighted. We understand that the retrospective nature of our study leads to concern about potential for error or bias. In contrast, we also realize it is unlikely this type of study could ever be replicated in a prospective manner due to continued advances in CMV prophylaxis strategies. In this study, we attempted to limit bias by specifying minimum predefined exclusions. Patients were excluded if there is a serious lack of CMV-DNA levels monitoring data. Indeed, very few patients were excluded due to missing data or lost to follow-up.
In summary, this study showed that the combination of CMV-IVIG and antiviral therapy is safe and effective for post-allo-HSCT CMV viremia and can accelerate CMV-DNA conversion. The regimen is especially indicated for haploidentical transplant recipients receiving high-dose ATG.
Acknowledgments
Funding: The Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019XK320076).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-1069
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-1069
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-1069). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol was approved by the Ethics Committee, Institute of Hematology & Hospital of Blood Diseases, Chinese Academy of Medical Sciences (HG2021009-EC-1). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chan ST, Logan AC. The clinical impact of cytomegalovirus infection following allogeneic hematopoietic cell transplantation: Why the quest for meaningful prophylaxis still matters. Blood Rev 2017;31:173-83. [Crossref] [PubMed]
- Cantoni N, Hirsch HH, Khanna N, et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant 2010;16:1309-14. [Crossref] [PubMed]
- Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 2019;19:e260-e272. [Crossref] [PubMed]
- Avery RK, Arav-Boger R, Marr KA, et al. Outcomes in Transplant Recipients Treated With Foscarnet for Ganciclovir-Resistant or Refractory Cytomegalovirus Infection. Transplantation 2016;100:e74-80. [Crossref] [PubMed]
- Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol 1997;97:855-64. [Crossref] [PubMed]
- Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21:389-401.e1. [Crossref] [PubMed]
- Reusser P, Einsele H, Lee J, et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood 2002;99:1159-64. [Crossref] [PubMed]
- Boppana SB, Britt WJ. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis 1995;171:1115-21. [Crossref] [PubMed]
- Nigro G, Adler SP, La Torre R, et al. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 2005;353:1350-62. [Crossref] [PubMed]
- Schoppel K, Schmidt C, Einsele H, et al. Kinetics of the antibody response against human cytomegalovirus-specific proteins in allogeneic bone marrow transplant recipients. J Infect Dis 1998;178:1233-43. [Crossref] [PubMed]
- Zdziarski P. CMV-Specific Immune Response-New Patients, New Insight: Central Role of Specific IgG during Infancy and Long-Lasting Immune Deficiency after Allogenic Stem Cell Transplantation. Int J Mol Sci 2019;20:271. [Crossref] [PubMed]
- Fisher RA, Kistler KD, Ulsh P, et al. The association between cytomegalovirus immune globulin and long-term recipient and graft survival following liver transplantation. Transpl Infect Dis 2012;14:121-31. [Crossref] [PubMed]
- Zamora D, Krantz EM, Green ML, et al. Cytomegalovirus Humoral Response Against Epithelial Cell Entry-Mediated Infection in the Primary Infection Setting After Hematopoietic Cell Transplantation. J Infect Dis 2020;221:1470-9. [Crossref] [PubMed]
- Alexander BT, Hladnik LM, Augustin KM, et al. Use of cytomegalovirus intravenous immune globulin for the adjunctive treatment of cytomegalovirus in hematopoietic stem cell transplant recipients. Pharmacotherapy 2010;30:554-61. [Crossref] [PubMed]
- Czer LS, Ruzza A, Vespignani R, et al. Prophylaxis of cytomegalovirus disease in mismatched patients after heart transplantation using combined antiviral and immunoglobulin therapy. Transplant Proc 2011;43:1887-92. [Crossref] [PubMed]
- Weill D, Lock BJ, Wewers DL, et al. Combination prophylaxis with ganciclovir and cytomegalovirus (CMV) immune globulin after lung transplantation: effective CMV prevention following daclizumab induction. Am J Transplant 2003;3:492-6. [Crossref] [PubMed]
- Zhang LN, Li MH, Zhou J, et al. Human cytomegalovirus pneumonia and intestinal acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: a case report and literatures review. Zhonghua Xue Ye Xue Za Zhi 2018;39:245-7. [PubMed]
- Wu JL, Ma HY, Lu CY, et al. Risk factors and outcomes of cytomegalovirus viremia in pediatric hematopoietic stem cell transplantation patients. J Microbiol Immunol Infect 2017;50:307-13. [Crossref] [PubMed]


