
Side effects of CDK4/6 inhibitors in the treatment of HR+/HER2− advanced breast cancer: a systematic review and meta-analysis of randomized controlled trials
Introduction
Currently, one of the most prevalent malignancies in women is breast cancer (BC) and it is the major cause of cancer death of women worldwide (1). Because almost 70% of patients have the subtype of hormone receptor (HR)-positive (+) or human epidermal growth factor receptor type (HER2)-negative (–) (2), endocrine therapy, also known as hormone therapy, is the widely used clinical regimen for efficacy and benign drug toxicity profile (3), specifically targeting the estrogen-receptor signaling pathway (4). In particular, endocrine therapy with an aromatase inhibitor (AI) has a vital role in treating HR(+) and HER2(–) postmenopausal patients suffering from locally advanced or metastatic BC (ABC) for long-term disease management compared with tamoxifen (5-7). Lately, positive outcomes from numerous randomized clinical trials support the use of AIs as a standard primary treatment modality; in particular, three AIs (letrozole, anastrozole, and exemestane) have demonstrated superior performance to tamoxifen regarding efficacy endpoints (8-10). However, in nearly all patients AI resistance inevitably occurs, so research into novel clinical methods to tackle endocrine resistance is needed (11-14).
The cyclin-dependent kinases belong to a large family of serine threonine kinases and are crucial protein kinases that coordinate sequences of the cell cycle. In particular, interactions between cyclin D and CDKs 4 and 6 (CDK4/6) are pivotal in terms of controlling the cell division cycle, so significant implications of the CDKs in the carcinogenesis and endocrine therapy resistance of BC are reasonable(15,16). Both preclinical research and clinical trials have demonstrated activity of CDK4/6 inhibitors (CDK4/6Is) (palbociclib, ribociclib, and abemaciclib) in HR(+) BC (13,14,17-20). Therefore, the U.S. FDA and other pharmaceutical regulatory authorities around the world have licensed the use of CDK4/6Is either in conjunction with endocrine therapy (palbociclib, ribociclib, abemaciclib) (13,14,17-20) or as single agents (abemaciclib) (12,21) for the primary treatment of HR(+) and HER2(–) ABC patients.
Preclinical research has confirmed that PFS is enhanced in both phase II and phase III trials utilizing the three available CDK4/6Is combined with endocrine therapy (13,22). For example, PALOMA-2, MONALEESA-2, and MONARCH-3 evaluated CDK4/6Is incorporated with AIs, MONALEESA-7 evaluated CDK4/6Is with either tamoxifen or an AI, and MONARCH-2 evaluated abemaciclib in conjunction with fulvestrant in female patients with HR+/HER2− ABC (13,18,23,24,25).
However, it has been reported in these clinical trials that CDK4/6Is combined with endocrine therapy also induced toxicities for patients, which include neutropenia, fatigue, anemia, febrile neutropenia and so on (26). Therefore, we reviewed phase III clinical trials and conducted a meta-analysis to evaluate CDK4/6Is combined with endocrine therapy compared with placebo and endocrine therapy for the management of BC.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/apm-21-1096).
Methods
Search strategy
Two of the authors conducted a comprehensive research of electronic databases (Medline (Via Pubmed), Embase, the Cochrane Library, ASCO meeting library database, San Antonio meeting abstract database, and ESMO meeting abstract database) from January 2010 to December 2019. Keywords used included breast neoplasm, breast cancer, breast tumor, CDK4/6 inhibitor, abemaciclib, ribociclib, palbociclib, endocrine therapy, aromatase inhibitor, letrozole, fulvestrant, and adverse effects. The research criteria were restricted to published English RCTs, in which subjects were allocated to an experimental versus control group. When similar publications were encountered, the researchers incorporated the most current results (corresponding to longer follow-up). The Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) statement was assisted in the selection and analysis process (27). Clinical trials in which subjects were randomly designated to CDK4/6 inhibitor or placebo group (or control) were selected.
Study selection
The inclusion criteria were: (I) phase III RCT double-blind, first-line therapy published in English; (II) pathological diagnosis of ABC patients with HR-positive or Her2-negative; (III) detailed pathological data, follow-up time and ≥ grade 3 adverse effects reported; (IV) relative risk or hazard ratio (HR) and 95% confidence interval (CI) reported or the data could be converted to corresponding values; (V) when similar publications were found, only the most up-to-date reports that incorporated complete clinical trial safety data were included.
The exclusion criteria included: (I) unclear diagnosis; (II) sample size was too small; (III) trials with incomplete data reported, low reliability; (IV) the trial’s data could not be extracted and the author(s) could not be contacted; and (V) only an article summary provided or conference assembly data material.
Data extraction
First, the two independent reviewers screened studies containing the relevant keywords in titles and abstracts. Second, studies conforming to the inclusion criteria were subjected to full text inspection and analysis to further determine their relevance and evaluate the quality of the research work.
Risk of bias assessment
The two reviewing authors evaluated the bias of the included literature using the Cochrane risk bias assessment tool to reach accord in any disparities. When necessary, they sought the advice of a third author (Figure 1).
Statistical analysis
The outcome of treatment was appraised through HRs with 95% CIs for time-to-event outcomes (PFS) and risk ratios (RRs) with a 95% CI for dichotomous outcomes. The I2 value was calculated, and used to determine statistical heterogeneity; an I2 value between 0%and 30% implies no heterogeneity, between 30% and 60% reflects moderate heterogeneity, and between 50% and 90% is significant heterogeneity. An I2 value of 75–100% denotes considerable heterogeneity. Each statistical assessment was two-tailed with P≤0.05 as the threshold statistical significance. Review Manager analytical software version 5.3 was used to calculate and analyze the data, and the results are presented as forest plots.
Results
Study selection
A total of 1,854 studies were identified by the initial search and further reduced to a list of 714 potentially eligible articles, of which 708 were excluded. Finally, 6 articles with 3,685 patients suffering from advanced breast cancer were included (Figure 2).
Characteristics of the included studies
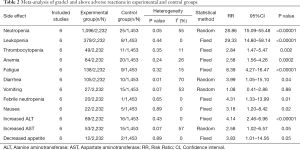
The characteristics of the six randomized phase III trials are listed in Table 1 (13,22). We performed a meta-analysis that compared experimental and control groups (Table 2).

Full table
Full table
Progression-free survival (PFS)
The meta-analysis discovered that in the trial group with CDK4/6 inhibitors, PFS was prolonged substantially (HR 0.54, 95% CI: 0.50–0.60, P<0.00001) in the absence of heterogeneity regarding this outcome (I2=0%) (Figure 3).
Neutropenia
All six studies reported neutropenia, and cumulative neutropenia increased significantly higher in the experimental group (RR 28.86, 95% CI: 15.01–55.48, P<0.00001), with heterogeneity (I2=55%, P=0.05) among the studies (Figure 4).
Leukopenia
All six studies reported leukopenia and the cumulative leukopenia rate substantially escalated in the experimental group (RR 29.33, 95% CI: 14.80–58.14, P<0.00001), with no heterogeneity (I2=0%, P=0.44) among the studies (Figure S1).
Thrombocytopenia
All six studies reported thrombocytopenia and the cumulative thrombocytopenia rate was significantly higher in the experimental group (RR 2.84, 95% CI: 1.47–5.47, P=0.002), with no heterogeneity (I2=0%, P=0.44) among the studies (Figure S2).
Anemia
All six studies reported anemia and the cumulative anemia rate was noticeably greater in the experimental group (RR 2.58, 95% CI: 1.56–4.26, P=0.0002), and no heterogeneity (I2=26%, P=0.24) among the studies (Figure S3).
Fatigue
All six studies reported fatigue and the cumulative fatigue rate was considerably higher in the experimental group (RR 8.39, 95% CI: 4.27–16.47, P=0.00001), with no heterogeneity (I2=15%, P=0.32) among the studies (Figure S4).
Diarrhea
Six studies reported diarrhea and the cumulative diarrhea rate was significantly higher in the experimental group (RR 3.99, 95% CI: 1.05–15.10, P=0.04), with heterogeneity (I2=70%, P=0.01) among the studies (Figure S5).
Vomiting
As for vomiting (RR 1.08, 95% CI: 0.41–2.86, P=0.15), heterogeneity existed across studies (I2=53%), and no meaningful dissimilarities between the experimental and control groups were detected (Figure S6).
Febrile neutropenia
All six studies reported febrile neutropenia and the cumulative febrile neutropenia rate was considerably greater in the experimental group (RR 4.31, 95% CI: 1.33–13.99, P=0.01), with no heterogeneity (I2=0%, P=0.01) among the studies (Figure S7).
Nausea
All six studies reported nausea and the cumulative nausea rate rose substantially in the experimental group (RR 3.18, 95% CI: 1.20–8.42, P=0.02), with no heterogeneity (I2=0%, P=0.89) among the studies (Figure S8).
Increased ALT
All six studies reported increased levels of ALT and the cumulative increased ALT rate was considerably higher in the experimental group (RR 4.14, 95% CI: 2.46–6.95, P<0.00001), with no heterogeneity (I2=0%, P=0.43) among the studies (Figure S9).
Increased AST
As for increased AST (RR 2.58, 95% CI: 1.02–6.57, P=0.05), heterogeneity existed across studies (I2=57%, P=0.07), and no significant variation between the experimental and control groups as observed (Figure S10).
Decreased appetite
No heterogeneity existed across studies (I2=0%, P=0.89) for decreased appetite (RR 3.83, 95% CI: 1.01–14.56, P=0.05), and no significant differences between the experimental and control groups were observed (Figure S11).
Publication bias
The publication bias was evaluated by funnel plot, and none was found.
Discussion
CDK4/6 inhibitors have been widely used for HR+ breast cancer, and combined with hormonal treatment has become the first-line standardized treatment option for both premenopausal and postmenopausal women. Importantly, in all first-line CDK4/6 inhibitors with hormonal treatment trials the ORRs of over 50% have been proven, and the result makes CDK4/6 inhibitors plus hormonal treatment become a suitable option over chemotherapy in most cases. CDK4/6 inhibitors are also becoming a standard option for these CDK4/6 inhibitor naïve patients who previously treated with endocrine therapy. Therefore, CDK4/6Is are the choice of drug treatment for patients suffering from advanced BC to improve PFS. A meta-analysis published in JAMA indicated that, compared with endocrine therapy alone, treatment with CDK4/6 inhibitors plus endocrine therapy was associated with significantly improved OS, PFS, and objective response rate among patients with HR(+), HER2(−) metastatic breast cancer.(28)CDK4/6 have strong specification in the treatment of BC because of their role in regulating the cell cycle. Studies of other tumors have also shown their therapeutic efficacy, but the side effects of inhibitors cannot be ignored (29). Through detailed evaluation of these side effects, CDK4/6Is can be better used in clinical practice. Most clinical studies focus on the grade3 and above adverse reactions of neutropenia, leucopenia, thrombocytopenia, anemia, diarrhea, vomiting, febrile neutropenia, nausea, increased ALT, increased AST, and decreased appetite. In the six phase III RCTs with double-blind, first-line therapy included in our meta-analysis, the occurrence of neutropenia, leukopenia, thrombocytopenia, anemia, fatigue, diarrhea, febrile neutropenia, nausea and increased ALT was substantially higher in all studies, while the incidence of vomiting, increased AST, and lack of appetite was not substantially different among the studies.
With the use of CDK4/6Is, the PFS of tumor patients has been significantly prolonged, but the occurrence of cardiotoxicity has gradually increased (30,31). Cardiac toxicity and the other side effects have a major effect on the quality of life of cancer patients and the death rate due to heart disease has increased significantly. At present, the potential side effects of CDK4/6Is on myocardial cells are still unclear. Because the heart does not have regenerative ability, long-term use of cardiotoxic drugs harmful will cause irreversible. Therefore, cardiac side effects are an important limitation on clinical use. There are few reports on the effect of CDK4/6Is on the heart, which requires further research. However, CDK4/6Is are gradually revolutionizing cancer therapy of HR+/HER2− ABC patients. As primary treatment, the proportion of CDK4/6Is has increased rapidly, yet the proportion of chemotherapy programs decreased modestly and the proportion of using selective estrogen receptor decline regulator alone gradually decreased. CDK4/6Is are really beneficial for the PFS of patients with HR+/HER2− ABC and can delay the start of chemotherapy. However, more clinical trial data are still needed to determine whether this therapy is beneficial or not. CDK4/6I combined with endocrine therapy can improve the effective rate and median PFS of patients with HR+/HER2− ABC, but this treatment regimen increases the incidence of adverse reactions such as neutropenia, leukopenia, thrombocytopenia, anemia, fatigue, diarrhea, febrile neutropenia, nausea and increased ALT.
The main concerns for HR+/HER2− ABC patients are whether CDK4/6 inhibitors can prolong their OS, and whether it has a role in continuing treatment beyond progression. These questions will be answered when more trial data are presented in the future. With our in-depth understanding of the pharmacological and molecular mechanisms of these drugs, we will put forward more refined questions, and ultimately find a more appropriate treatment scheme, which can benefit the majority breast cancer patients and reduce their unnecessary toxicity and costs.
Conclusions
For HR+ breast cancer patients, endocrine therapy is the basis, but endocrine resistance has been a critical clinical problem. The CDK4/6 inhibitors have effectively improved the survival of HR+/HER2-ABC patients with tolerable adverse effects, especially endocrine resistance can be reversed in some event when CDK4/6 inhibitors combined with endocrine therapy, Palbociclib, ribociclib, and abemaciclib have changed the treatment pattern of hormone receptor-positive ABC and has become the new standard of treatment.
CDK 4/6Is can restore the normal cell cycle, trigger anti-tumor immunity, and change the tumor microenvironment. Alone or in combination, they are used for the treatment of BC, lung cancer, liver cancer, pancreatic cancer and other cancers and have achieved certain curative effects. They inhibit the proliferation and development of malignant tumors and in combination with other anti-tumor drugs can effectively reduce the emergence of drug resistance and synergistically enhance clinical efficacy. Endocrine therapy, with its good efficacy and safety, is an important treatment for patients with hormone receptor-positive progressive BC and generally recommended more often for systemic therapy than chemotherapy to improve disease control and prolong survival. CDK4/6Is also have unique advantages in the treatment of BC, in that although they may cause side effects, especially hematological changes, non-hematological toxicity is less severe.
In recent years, there has been rapid progress in the field of endocrine therapy. CDK4/6Is combined with endocrine therapy can bring survival benefits to patients with hormone receptor-positive progressive BC, which then delays the timing of chemotherapy. The treatment of hormone receptor-positive progressive BC is gradually changing. The latest literature reports the discovery of abemaciclib derivatives in cardiomyocytes activating the hippo signaling pathway (12,21). Understanding the molecular basis of the cardiac side effects of chemotherapeutic drugs can lead to effective prevention and treat of cancer drug-induced heart disease.
Although a large number of studies of CDK4/6Is show that PFS, ORR and CBR have obviously improved, through this meta-analysis we focused on other side effects that significantly diminish patients’ quality of life. In addition to prolonging survival, reducing side effects as much as possible and bringing better quality of life to patients cannot be ignored. How to maintain the balance between the higher survival rate and the decline in quality of life is the direction of future clinical research. Furthermore, besides endocrine therapy, exploring CDK4/6 inhibitor in combination with targeted therapy, chemotherapy, immunotherapy, and radiotherapy have emerged in more and more laboratories and clinical trials. It indicates that the use of CDK4/6 inhibitors dose not limited to HR+/HER2− ABC patients.
It is hoped that more basic and clinical studies will continue to explore the precise beneficiary populations of CDK4/6 inhibitors in the future, in order to obtain better clinical effects from combined treatment strategy formulated from multiple angles, such as signaling pathways and regulation of the tumor immune microenvironment.
Study limitations
Because CDK4/6Is are a new class of drug, the number of related studies is still relatively small, which may affect the accuracy of the results. Non-English literature was not included, which may lead to publication bias. The drugs used in the studies were not completely identical, leading to clinical heterogeneity among the studies. Due to the short research time, many outcome indicators have not been reported and therefore could not be analyzed, and long-term efficacy needs further evaluation.
Acknowledgments
We acknowledge the reviewers for their helpful comments.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-1096
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-1096). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Tryfonidis K, Zardavas D, Katzenellenbogen BS, et al. Endocrine treatment in breast cancer: Cure, resistance and beyond. Cancer Treat Rev 2016;50:68-81. [Crossref] [PubMed]
- Rugo HS, Rumble RB, Macrae E, et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 2016;34:3069-103. [Crossref] [PubMed]
- Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol 2013;8:135-55. [Crossref] [PubMed]
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2)†. Ann Oncol 2014;25:1871-88. [Crossref] [PubMed]
- Migliaccio I, Malorni L, Hart CD, et al. Endocrine therapy considerations in postmenopausal patients with hormone receptor positive, human epidermal growth factor receptor type 2 negative advanced breast cancers. BMC Med 2015;13:46. [Crossref] [PubMed]
- Joy AA, Ghosh M, Fernandes R, Clemons MJ. Systemic treatment approaches in her2-negative advanced breast cancer-guidance on the guidelines. Curr Oncol 2015;22:S29-42. [Crossref] [PubMed]
- Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 2000;18:3758-67. [Crossref] [PubMed]
- Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol 2008;26:4883-90. [Crossref] [PubMed]
- Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003;21:2101-9. [Crossref] [PubMed]
- Milani A, Geuna E, Mittica G, Valabrega G. Overcoming endocrine resistance in metastatic breast cancer: Current evidence and future directions. World J Clin Oncol 2014;5:990-1001. [Crossref] [PubMed]
- Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast Cancer. Clin Cancer Res 2017;23:5218-24. [Crossref] [PubMed]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-36. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med 2016;375:1738-48. [Crossref] [PubMed]
- Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov 2016;6:353-67. [Crossref] [PubMed]
- Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 2016;18:17. [Crossref] [PubMed]
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25-35. [Crossref] [PubMed]
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol 2017;35:3638-46. [Crossref] [PubMed]
- Rugo HS, Diéras V, Gelmon KA, et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol 2018;29:888-94. [Crossref] [PubMed]
- Turner NC, Finn RS, Martin M, et al. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol 2018;29:669-80. [Crossref] [PubMed]
- . Correction: MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast Cancer. Clin Cancer Res 2018;24:5485. [Crossref] [PubMed]
- Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425-39. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 2018;29:1541-7. [Crossref] [PubMed]
- Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018;19:904-15. [Crossref] [PubMed]
- Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in Combination WithFulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol 2017;35:2875-84. [Crossref] [PubMed]
- Spring LM, Zangardi ML, Moy B, et al. Clinical Management of Potential Toxicities and Drug Interactions Related to Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: Practical Considerations and Recommendations. Oncologist 2017;22:1039-48. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Li J, Huo X, Zhao F, et al. Association of Cyclin-Dependent Kinases 4 and 6 Inhibitors With Survival in Patients With Hormone Receptor-Positive Metastatic Breast Cancer A Systematic Review and Meta-analysis. JAMA Netw Open 2020;3:e2020312 [Crossref] [PubMed]
- Turner NC, Ro J, André F, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med 2015;373:209-19. [Crossref] [PubMed]
- Zhou Y, Li Y, Shen J, et al. Abemaciclib induces apoptosis in cardiomyocytes by activating the Hippo signaling pathway. Acta Biochim Biophys Sin (Shanghai) 2020;52:875-82. [Crossref] [PubMed]
- Guha A, Armanious M. Update on cardio-oncology: Novel cancer therapeutics and associated cardiotoxicities. Trends in cardiovascular medicine 2019;29:29-39. [Crossref] [PubMed]
(English Language Editor: K. Brown)






